Chapter 2 Isomerism Isomerism Isomers are compounds that
- Slides: 15

Chapter 2 Isomerism

Isomerism Isomers are compounds that have the same formula but different structures Structural Isomerism 1 - Skeletal Isomerism 2 - Positional Isomerism 3 - Functional Isomerism 4 - Tutomeric Isomerism Stereo Isomerism 1 - Geometric Isomerism

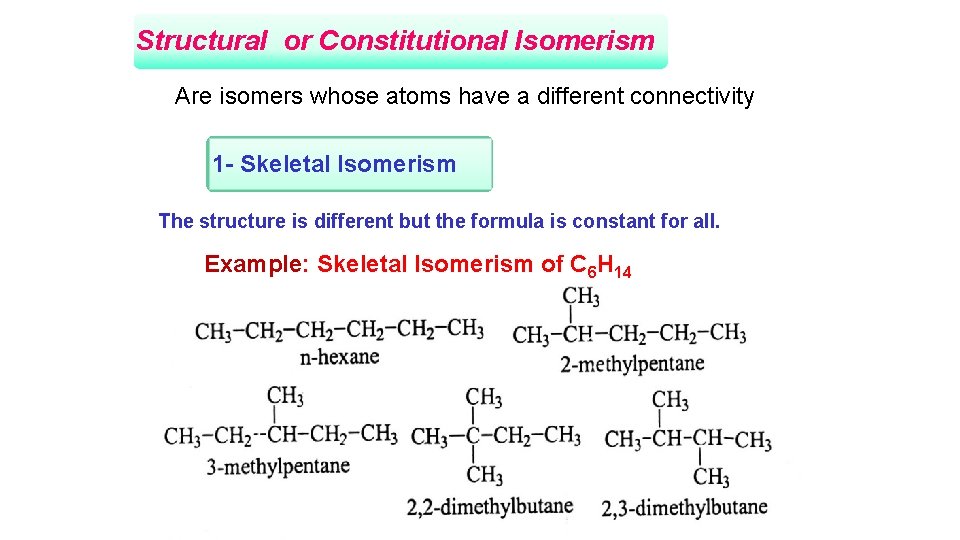
Structural or Constitutional Isomerism Are isomers whose atoms have a different connectivity 1 - Skeletal Isomerism The structure is different but the formula is constant for all. Example: Skeletal Isomerism of C 6 H 14

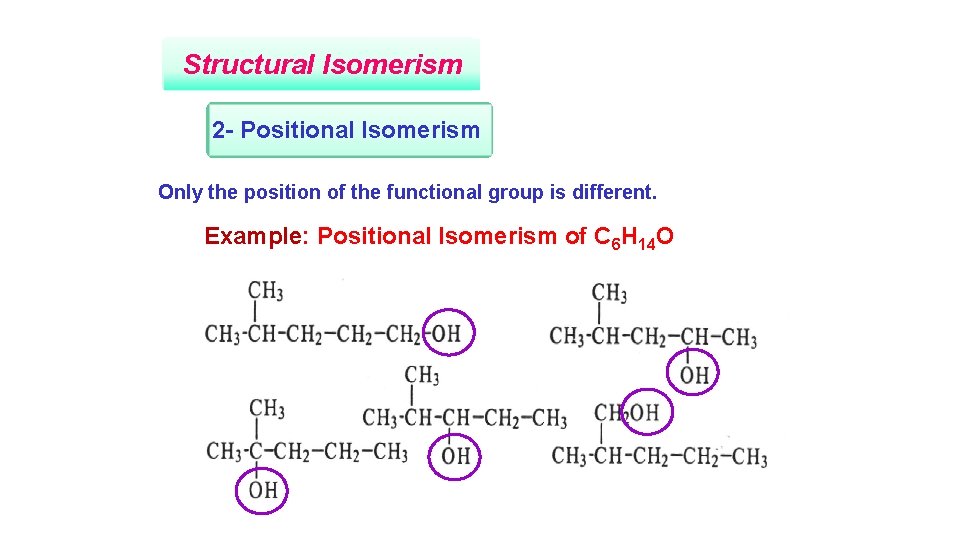
Structural Isomerism 2 - Positional Isomerism Only the position of the functional group is different. Example: Positional Isomerism of C 6 H 14 O

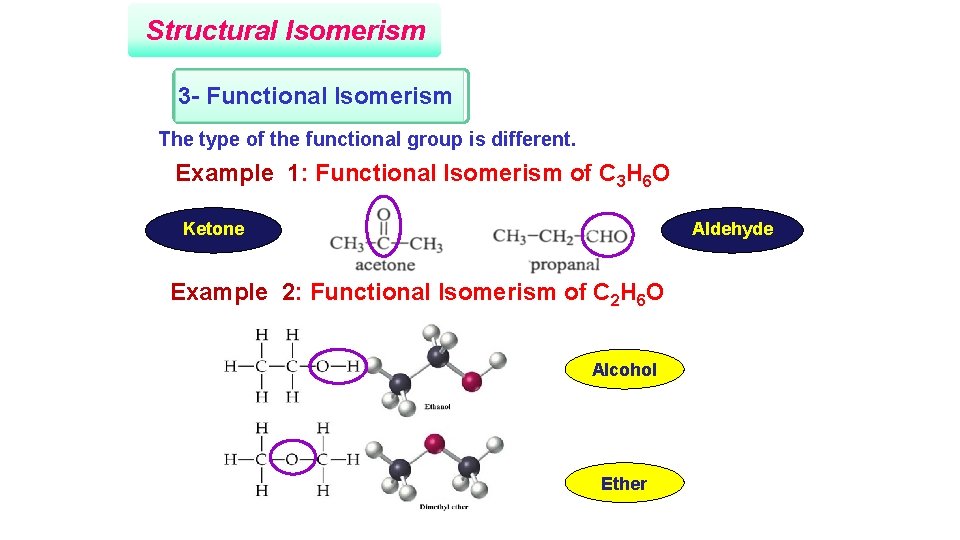
Structural Isomerism 3 - Functional Isomerism The type of the functional group is different. Example 1: Functional Isomerism of C 3 H 6 O Ketone Aldehyde Example 2: Functional Isomerism of C 2 H 6 O Alcohol Ether

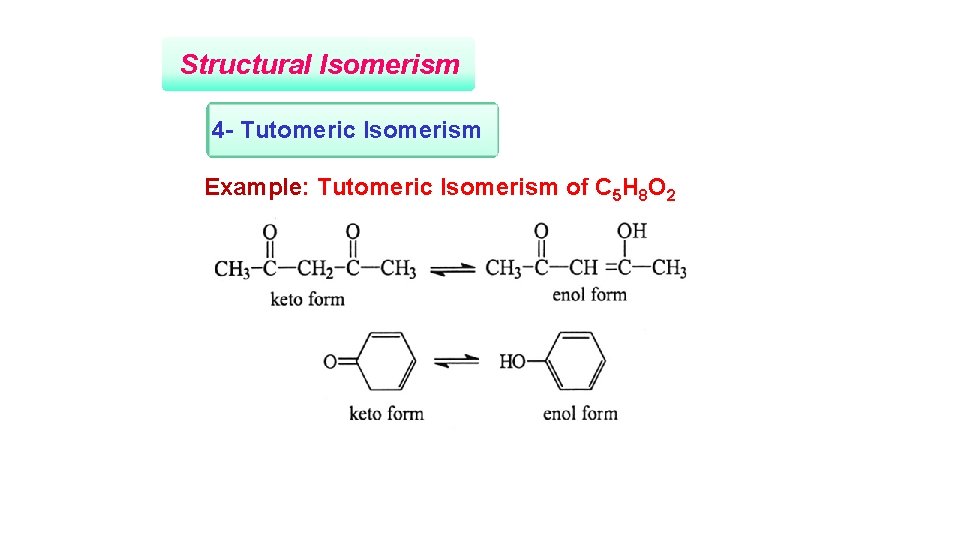
Structural Isomerism 4 - Tutomeric Isomerism Example: Tutomeric Isomerism of C 5 H 8 O 2

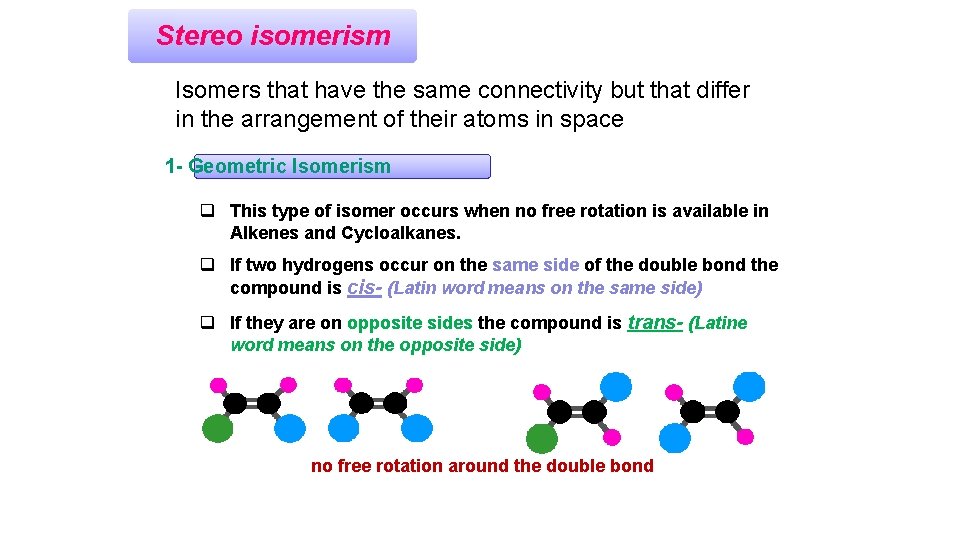
Stereo isomerism Isomers that have the same connectivity but that differ in the arrangement of their atoms in space 1 - Geometric Isomerism q This type of isomer occurs when no free rotation is available in Alkenes and Cycloalkanes. q If two hydrogens occur on the same side of the double bond the compound is cis- (Latin word means on the same side) q If they are on opposite sides the compound is trans- (Latine word means on the opposite side) no free rotation around the double bond

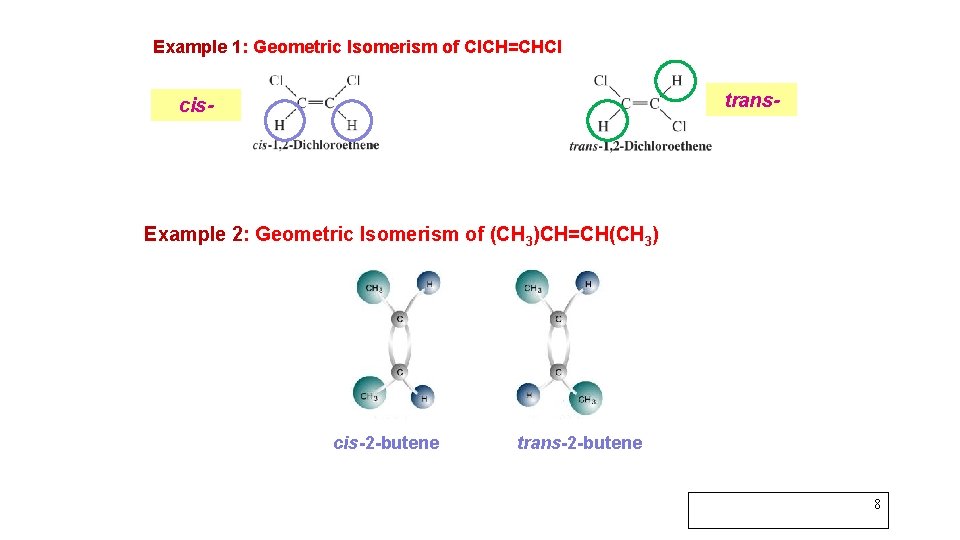
Example 1: Geometric Isomerism of Cl. CH=CHCl trans- cis- Example 2: Geometric Isomerism of (CH 3)CH=CH(CH 3) cis-2 -butene trans-2 -butene 8

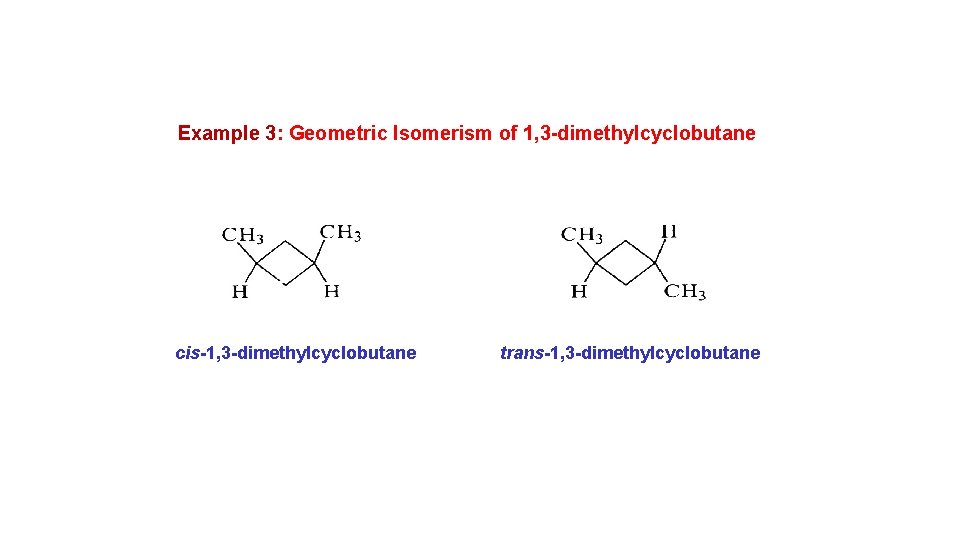
Example 3: Geometric Isomerism of 1, 3 -dimethylcyclobutane cis-1, 3 -dimethylcyclobutane trans-1, 3 -dimethylcyclobutane

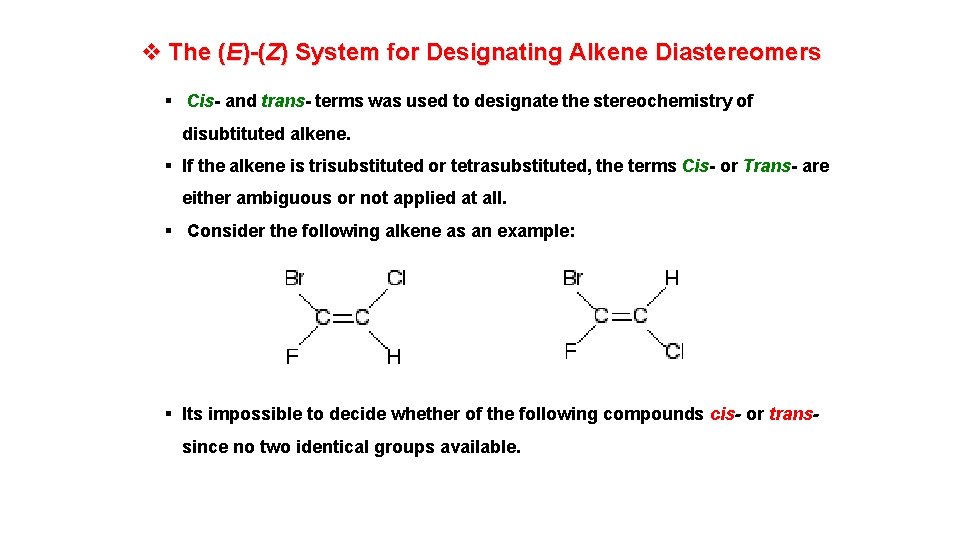
v The (E)-(Z) System for Designating Alkene Diastereomers § Cis- and trans- terms was used to designate the stereochemistry of disubtituted alkene. § If the alkene is trisubstituted or tetrasubstituted, the terms Cis- or Trans- are either ambiguous or not applied at all. § Consider the following alkene as an example: § Its impossible to decide whether of the following compounds cis- or transsince no two identical groups available.

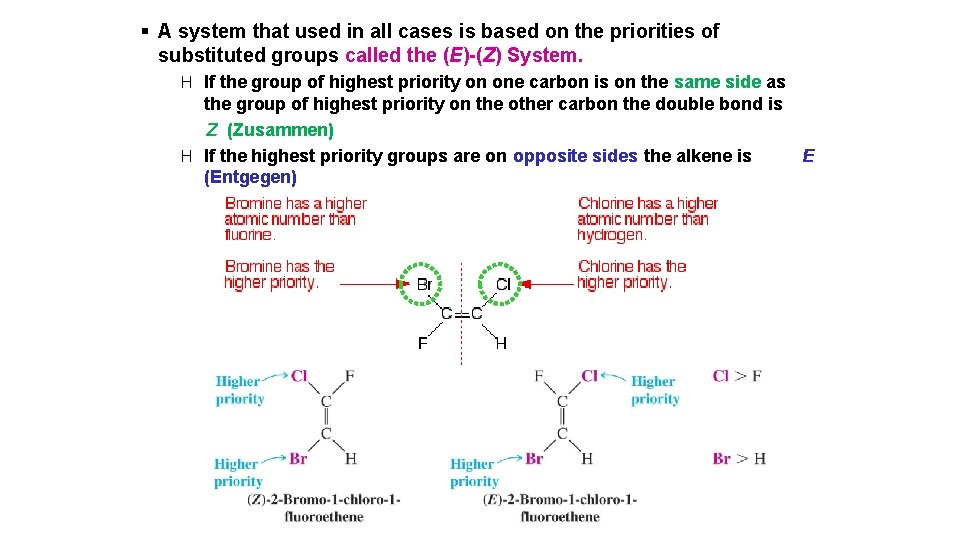
§ A system that used in all cases is based on the priorities of substituted groups called the (E)-(Z) System. H If the group of highest priority on one carbon is on the same side as the group of highest priority on the other carbon the double bond is Z (Zusammen) H If the highest priority groups are on opposite sides the alkene is (Entgegen) E

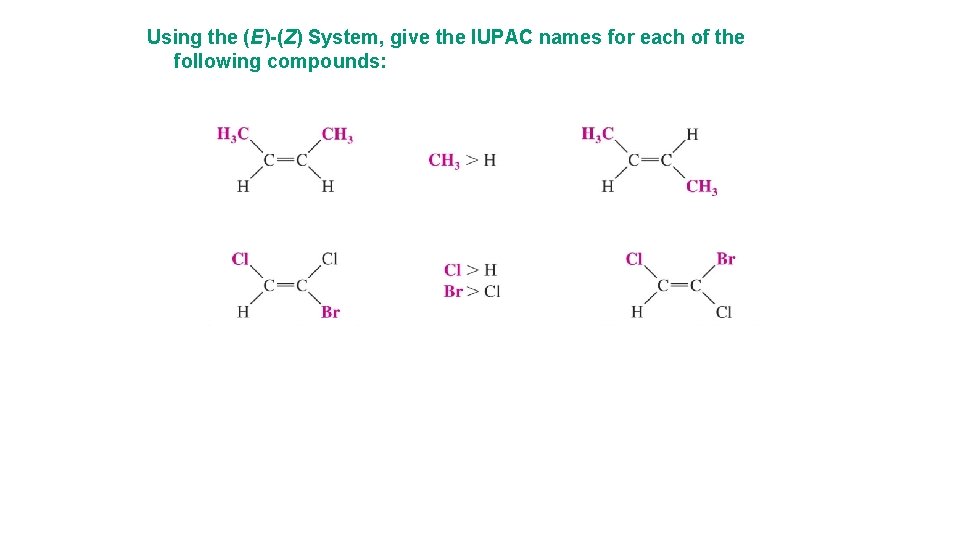
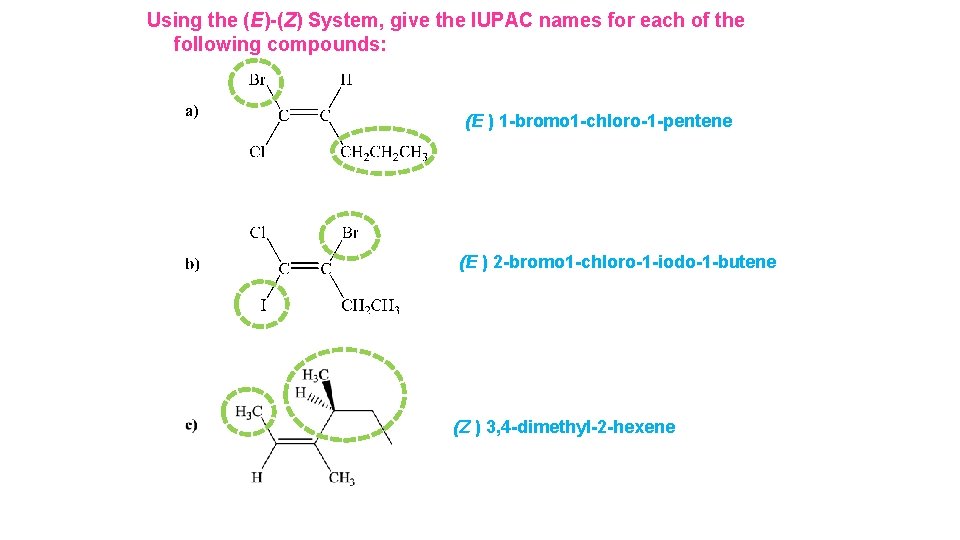
Using the (E)-(Z) System, give the IUPAC names for each of the following compounds:

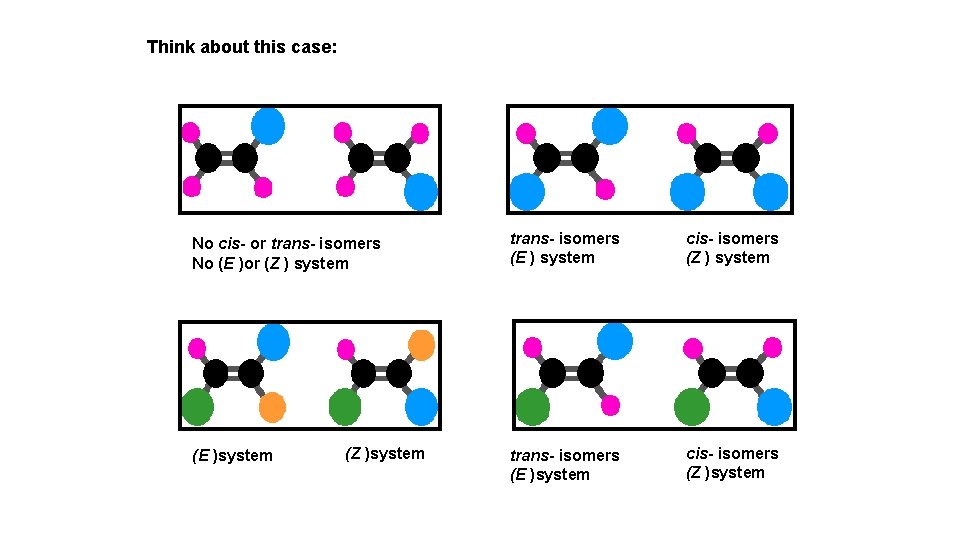
Think about this case: No cis- or trans- isomers No (E )or (Z ) system (E )system (Z )system trans- isomers (E ) system cis- isomers (Z ) system trans- isomers (E )system cis- isomers (Z )system

Using the (E)-(Z) System, give the IUPAC names for each of the following compounds: (E ) 1 -bromo 1 -chloro-1 -pentene (E ) 2 -bromo 1 -chloro-1 -iodo-1 -butene (Z ) 3, 4 -dimethyl-2 -hexene

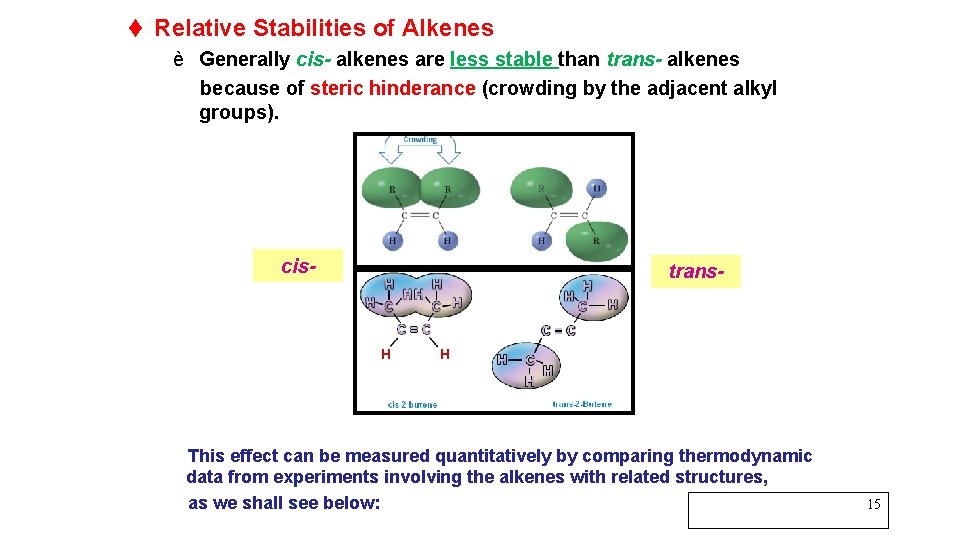
t Relative Stabilities of Alkenes è Generally cis- alkenes are less stable than trans- alkenes because of steric hinderance (crowding by the adjacent alkyl groups). cis- trans- This effect can be measured quantitatively by comparing thermodynamic data from experiments involving the alkenes with related structures, as we shall see below: 15