Optical Isomerism Optical isomerism arises in organic molecules
- Slides: 3

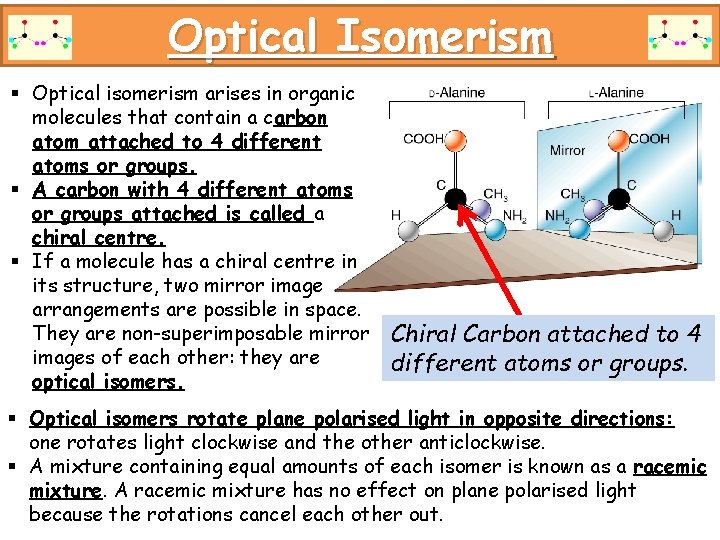
Optical Isomerism § Optical isomerism arises in organic molecules that contain a carbon atom attached to 4 different atoms or groups. § A carbon with 4 different atoms or groups attached is called a chiral centre. § If a molecule has a chiral centre in its structure, two mirror image arrangements are possible in space. They are non-superimposable mirror Chiral Carbon attached to 4 images of each other: they are different atoms or groups. optical isomers. § Optical isomers rotate plane polarised light in opposite directions: one rotates light clockwise and the other anticlockwise. § A mixture containing equal amounts of each isomer is known as a racemic mixture. A racemic mixture has no effect on plane polarised light because the rotations cancel each other out.

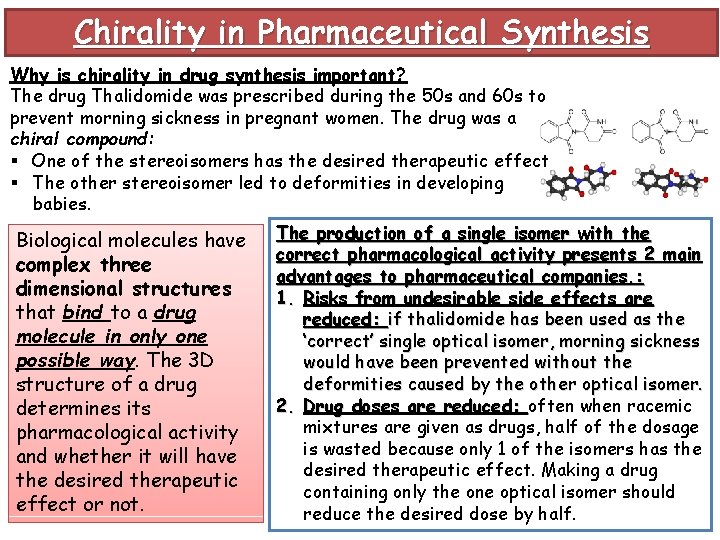
Chirality in Pharmaceutical Synthesis Why is chirality in drug synthesis important? The drug Thalidomide was prescribed during the 50 s and 60 s to prevent morning sickness in pregnant women. The drug was a chiral compound: § One of the stereoisomers has the desired therapeutic effect § The other stereoisomer led to deformities in developing babies. Biological molecules have complex three dimensional structures that bind to a drug molecule in only one possible way. The 3 D structure of a drug determines its pharmacological activity and whether it will have the desired therapeutic effect or not. The production of a single isomer with the correct pharmacological activity presents 2 main advantages to pharmaceutical companies. : 1. Risks from undesirable side effects are reduced: if thalidomide has been used as the ‘correct’ single optical isomer, morning sickness would have been prevented without the deformities caused by the other optical isomer. 2. Drug doses are reduced: often when racemic mixtures are given as drugs, half of the dosage is wasted because only 1 of the isomers has the desired therapeutic effect. Making a drug containing only the one optical isomer should reduce the desired dose by half.

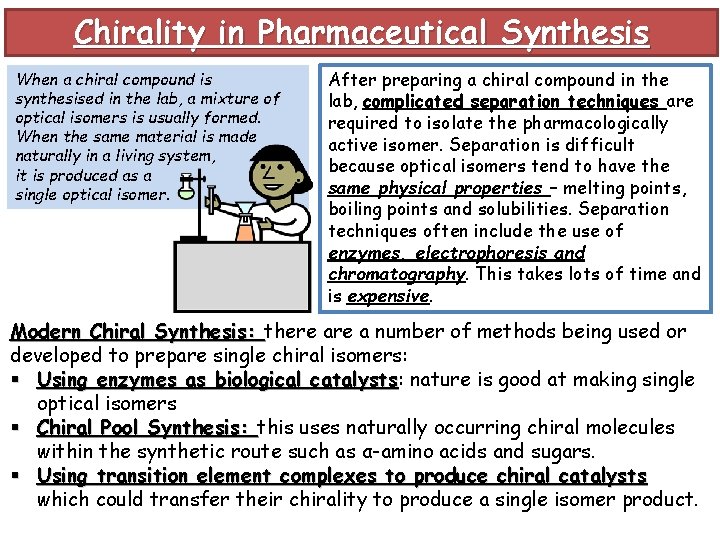
Chirality in Pharmaceutical Synthesis When a chiral compound is synthesised in the lab, a mixture of optical isomers is usually formed. When the same material is made naturally in a living system, it is produced as a single optical isomer. After preparing a chiral compound in the lab, complicated separation techniques are required to isolate the pharmacologically active isomer. Separation is difficult because optical isomers tend to have the same physical properties – melting points, boiling points and solubilities. Separation techniques often include the use of enzymes, electrophoresis and chromatography. This takes lots of time and is expensive. Modern Chiral Synthesis: there a number of methods being used or developed to prepare single chiral isomers: § Using enzymes as biological catalysts: catalysts nature is good at making single optical isomers § Chiral Pool Synthesis: this uses naturally occurring chiral molecules within the synthetic route such as α-amino acids and sugars. § Using transition element complexes to produce chiral catalysts which could transfer their chirality to produce a single isomer product.