CHAPTER 2 CHEMICAL FORMULAS COMPOSITION STOICHIOMETRY CHEMICAL FORMULAS



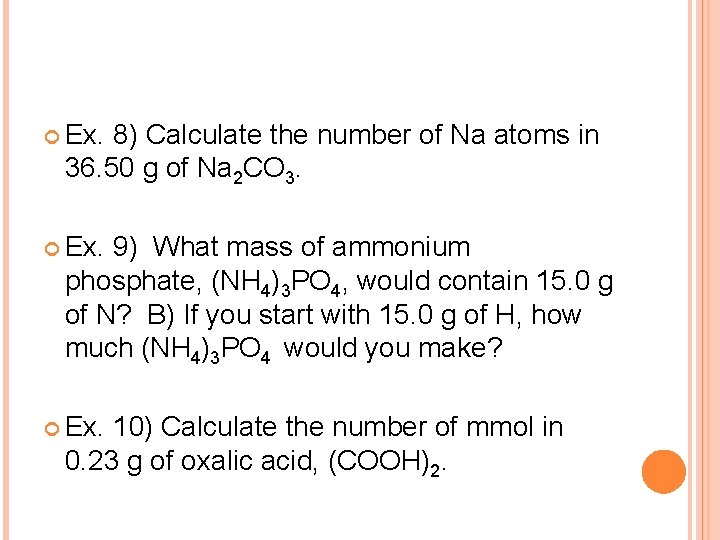

- Slides: 17

CHAPTER 2 CHEMICAL FORMULAS & COMPOSITION STOICHIOMETRY

CHEMICAL FORMULAS show the ratio of the elements present in the molecule or compound He, Au, Na - monatomic O 2, H 2, Cl 2 - diatomic O 3, P 4, S 8 - more complex elements H 2 O, C 12 H 22 O 11 – compounds ~ contains 2 or more elements Allotropes ~ different forms of the same element in the same physical state. Ex. Both O 2 and O 3 are gases

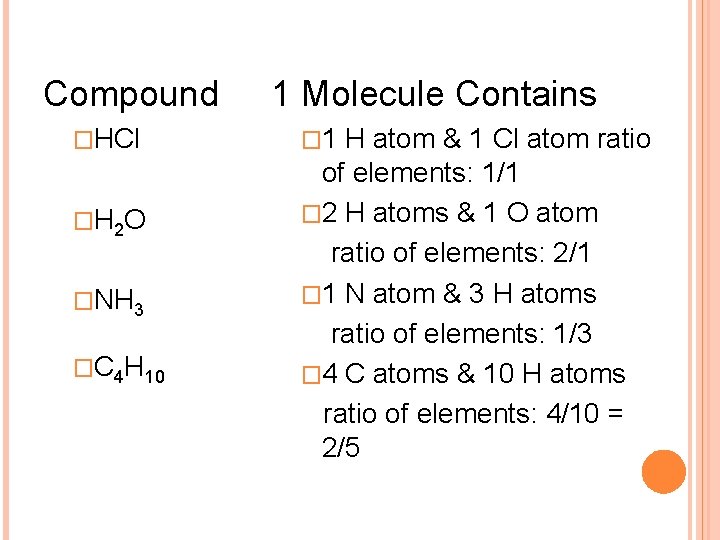
Compound �HCl �H 2 O �NH 3 �C 4 H 10 1 Molecule Contains � 1 H atom & 1 Cl atom ratio of elements: 1/1 � 2 H atoms & 1 O atom ratio of elements: 2/1 � 1 N atom & 3 H atoms ratio of elements: 1/3 � 4 C atoms & 10 H atoms ratio of elements: 4/10 = 2/5

IONS & IONIC COMPOUNDS ions are atoms or groups of atoms with an electrical charge two basic types of ions � positive ions or cations (+) one or more electrons less than neutral, ex. Na+ � negative ions or anions (-) one or more electrons more than neutral, ex. Cl- cations + anions must give neutral charge � KOH � Ca. SO 4 � Sr 3 N 2 � Al(OH)3 potassium hydroxide calcium sulfate strontium nitride aluminum hydroxide (+1 & -1) (+2 & -2) ((2 x 3)&(-3 x 2)) (+3 & (-1 x 3))

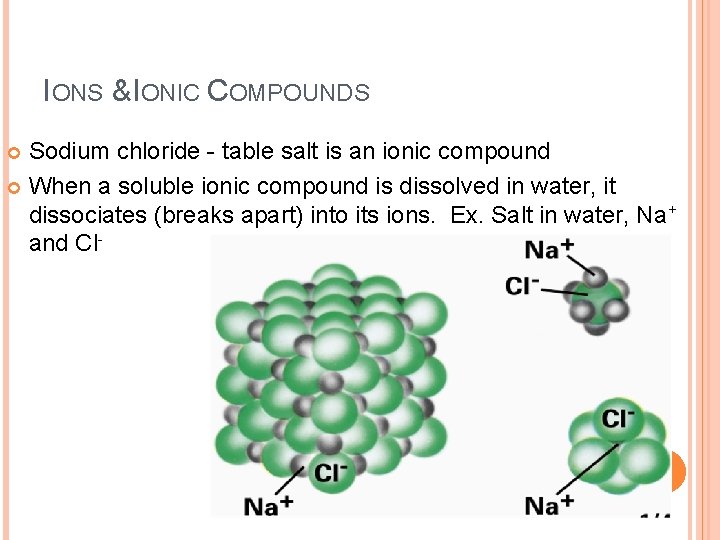
IONS & IONIC COMPOUNDS Sodium chloride - table salt is an ionic compound When a soluble ionic compound is dissolved in water, it dissociates (breaks apart) into its ions. Ex. Salt in water, Na+ and Cl

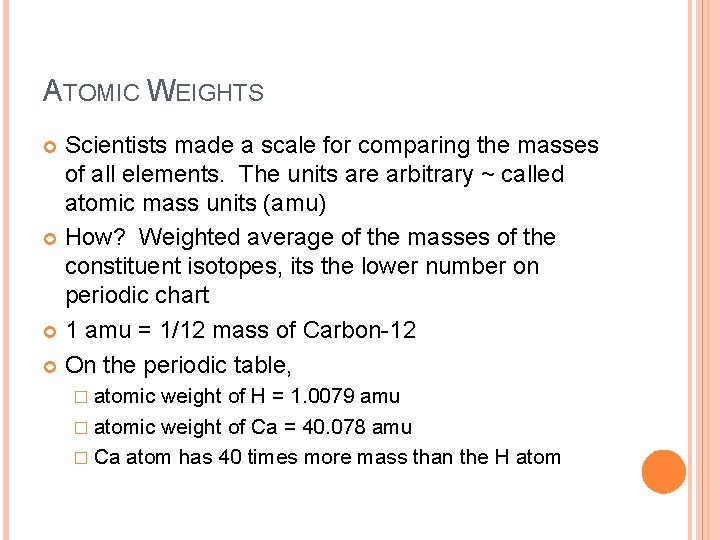
ATOMIC WEIGHTS Scientists made a scale for comparing the masses of all elements. The units are arbitrary ~ called atomic mass units (amu) How? Weighted average of the masses of the constituent isotopes, its the lower number on periodic chart 1 amu = 1/12 mass of Carbon-12 On the periodic table, � atomic weight of H = 1. 0079 amu � atomic weight of Ca = 40. 078 amu � Ca atom has 40 times more mass than the H atom

THE MOLE Atoms are very small, difficult to weigh and count, so how can we measure them accurately? Tha MOLE!!! strictly a convenience unit � amount that is large enough to see and handle in lab mole = number of things � dozen = 12 things � mole = 6. 022 x 1023 things Avogadro’s number = 6. 022 x 1023

The atomic weight (amu) = mass of 1 mol (g), also called molar mass - mass in grams equal to the atomic weight � Units of atomic weight are amu/atoms or g/mol � He exists as atoms: 4. 0026 g of He atoms = 1 mol He = 6. 022 x 10 23 atoms He � H 2 exists as molecules: 2. 0158 g of H 2 molecules= 1 mol H 2 = 6. 022 x 1023 molecules H 2 = 2 (6. 022 x 1023)atoms of H = 1. 204 x 1024 atoms of H

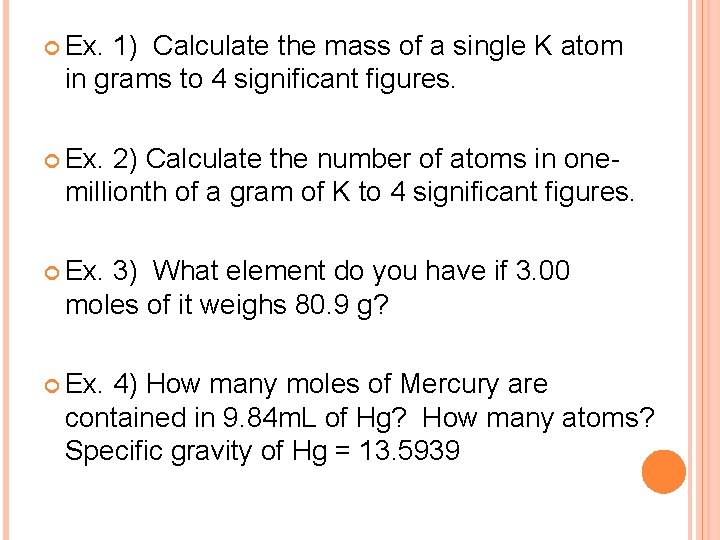
Ex. 1) Calculate the mass of a single K atom in grams to 4 significant figures. Ex. 2) Calculate the number of atoms in onemillionth of a gram of K to 4 significant figures. Ex. 3) What element do you have if 3. 00 moles of it weighs 80. 9 g? Ex. 4) How many moles of Mercury are contained in 9. 84 m. L of Hg? How many atoms? Specific gravity of Hg = 13. 5939

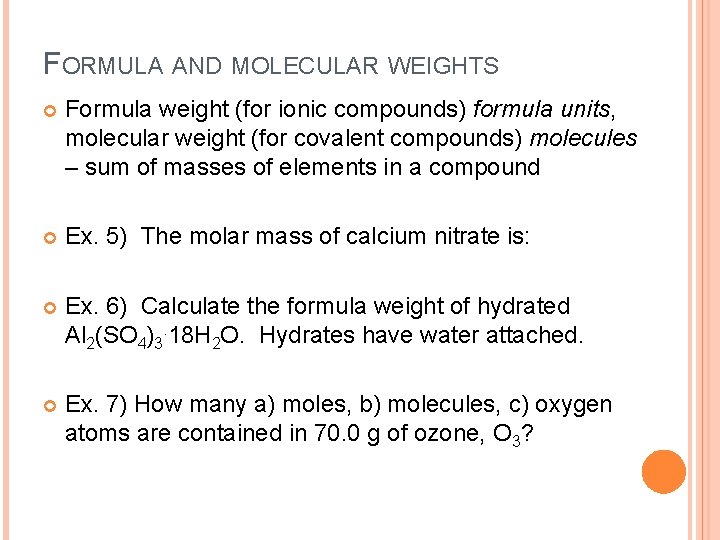
FORMULA AND MOLECULAR WEIGHTS Formula weight (for ionic compounds) formula units, molecular weight (for covalent compounds) molecules – sum of masses of elements in a compound Ex. 5) The molar mass of calcium nitrate is: Ex. 6) Calculate the formula weight of hydrated Al 2(SO 4)3. 18 H 2 O. Hydrates have water attached. Ex. 7) How many a) moles, b) molecules, c) oxygen atoms are contained in 70. 0 g of ozone, O 3?
Ex. 8) Calculate the number of Na atoms in 36. 50 g of Na 2 CO 3. Ex. 9) What mass of ammonium phosphate, (NH 4)3 PO 4, would contain 15. 0 g of N? B) If you start with 15. 0 g of H, how much (NH 4)3 PO 4 would you make? Ex. 10) Calculate the number of mmol in 0. 23 g of oxalic acid, (COOH)2.

PERCENT COMPOSITION mass of each element divided by the mass of the whole compound x 100% Ex. 11) Find the % by mass of the elements in hydrated Fe. SO 4. 7 H 2 O. Assume you have 1 mole of the compound. All samples of hydrated iron(II) sulfate have this composition b/c of Law of Definite Proportions which states that different samples of a pure compound contains the same elements in the same proportions.

EMPIRICAL & MOLECULAR FORMULAS Many times in the lab, a chemist will synthesize a compound. To help prove what was made the compound is sent for elemental analysis. From this the simplest formula is found. empirical formula - simplest molecular formula, shows ratios of elements but not actual numbers of elements molecular formula - actual numbers of atoms of each element in the compound determine empirical & molecular formulas of a compound from percent composition � percent composition is determined experimentally

EMPIRICAL & MOLECULAR FORMULAS Ex. of molecular and empirical formulas � Molecular C 6 H 6 P 4 O 10 SO 2 � Ex. Empirical CH P 2 O 5 SO 2 (same) 12) A compound is found to contain 85. 63% C and 14. 37% H by mass, what is it’s empirical formula? In another experiment its molar mass is found to be 56. 1 g/mol. What is its molecular formula?

PURITY OF SAMPLES The percent purity of a sample of a substance is always represented as % purity = mass of pure substance x 100% mass of sample ~ mass of sample includes impurities (works just like percentages) Ex. 13) A bottle of sodium phosphate, Na 3 PO 4, is 92. 3% pure Na 3 PO 4. What are the masses of Na 3 PO 4 and impurities in 250. g of this sample of Na 3 PO 4?

In 1986, Bednorz and Muller succeeded in making the first of a series of chemical compounds that were superconducting at relatively high temperatures. This first compound was La 2 Cu. O 4 which superconducts at 35 K. In their initial experiments, Bednorz and Muller made only a few mg of this material. How many La atoms are present in 3. 56 mg of La 2 Cu. O 4?

Within a year after Bednorz and Muller’s initial discovery of high temperature superconductors, Wu and Chu had discovered a new compound, YBa 2 Cu 3 O 7, that began to superconduct at 100 K. If we wished to make 1. 00 pound of YBa 2 Cu 3 O 7, how many grams of yttrium must we buy?