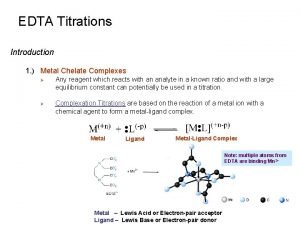
Chapter 11 Titrations Taking Advantage of Stoichiometric Reactions






- Slides: 18

Chapter 11 Titrations: Taking Advantage of Stoichiometric Reactions

n Titrimetric methods include a large and powerful group of quantitative procedures based on measuring the amount of a reagent of known concentration that is consumed by the analyte. Titrimetry is a term which includes a group of analytical methods based on determining the quantity of a reagent of known concentration that is required to react completely with the analyte.

n There are three main types of titrimetry: volumetric titrimetry, gravimetric titrimetry, and coulometrtic titrimetry. n Volumetric titrimetry is used to measure the volume of a solution of n Gravimetric titrimetry is like volumetric titrimetry, but the mass is measured instead of the volume. n Coulometric titrimetry is where the reagent is a constant direct electrical n The benefits of these methods are that they are rapid, accurate, convenient, and readily available. known concentration that is needed to react completely with the analyte. current of known magnitude that consumes the analyte; the time required to complete the electrochemical reaction is measured.

Defining Terms n n Standard Solution Titration Equivalence Point Back- Titration

Defining terms § Standard Solution: A reagent of a known concentration which is used in the titrimetric analysis. § Titration: This is performed by adding a standard solution from a buret or other liquid- dispensing device to a solution of the analyte until the point at which the reaction is believed to be complete.

More Defining Terms § Equivalence Point: Occurs in a titration at the point in which the amount of added titrant is chemically equivalent to the amount of analyte in a sample. § Back- Titration: This is a process that is sometimes necessary in which an excess of the standard titrant is added, and the amount of the excess is determined by back titration with a second standard titrant. In this instance the equivalence point corresponds with the amount of initial titrant is chemically equivalent to the amount of analyte plus the amount of back- titrant.

Equivalence Points and End Points One can only estimate the equivalence point by observing a physical change associated with the condition of equivalence. § End point: The point in titration when a physical change occurs that is associated with the condition of chemical equivalence. n Indicators are used to give an observable physical change (end point) at or near the equivalence point by adding them to the analyte. The difference between the end point and equivalence point should be very small and this difference is referred to as titration error. To determine the titration error: Et= Vep - Veq Et is the titration error n Vep is the actual volume used to get to the end point n Veq is theoretical value of reagent required to reach the end point n

Primary Standards n A primary standard is a highly purified compound that serves as a reference material in all volumetric and mass titrimetric properties. The accuracy depends on the properties of a compound and the important properties are: n 1. High purity n 2. Atmospheric stability n 3. Absence of hydrate water n 4. Readily available at a modest cost n 5. Reasonable solution in the titration medium n 6. Reasonably large molar mass Compounds that meet or even approach these criteria are few, and only a few primary standards are available.

Standard Solutions Standard solutions play a key role in titrimetric methods. Desirable Properties of Standard Solutions: 1. Sufficiently stable 2. React rapidly with analyte 3. React completely with analyte 4. Endure a selective reaction with analyte

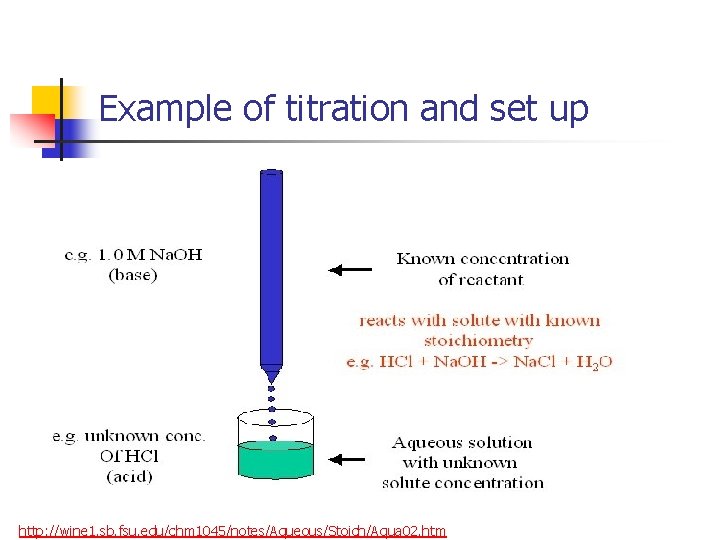
Example of titration and set up http: //wine 1. sb. fsu. edu/chm 1045/notes/Aqueous/Stoich/Aqua 02. htm

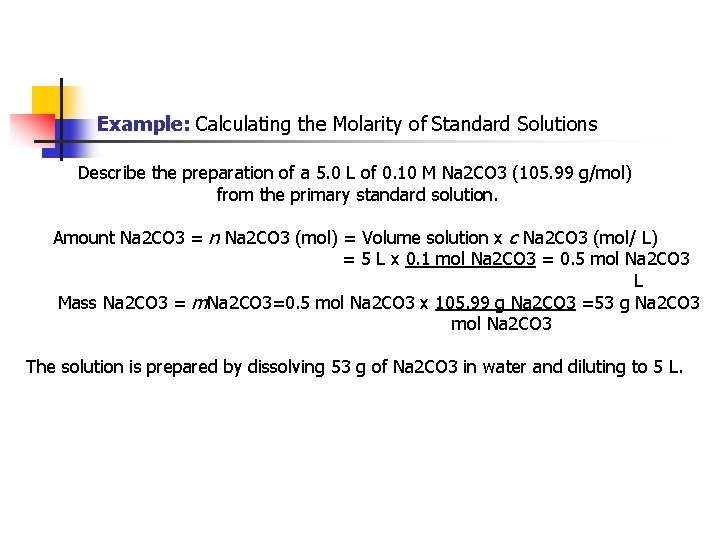
Example: Calculating the Molarity of Standard Solutions Describe the preparation of a 5. 0 L of 0. 10 M Na 2 CO 3 (105. 99 g/mol) from the primary standard solution. Amount Na 2 CO 3 = n Na 2 CO 3 (mol) = Volume solution x c Na 2 CO 3 (mol/ L) = 5 L x 0. 1 mol Na 2 CO 3 = 0. 5 mol Na 2 CO 3 L Mass Na 2 CO 3 = m. Na 2 CO 3=0. 5 mol Na 2 CO 3 x 105. 99 g Na 2 CO 3 =53 g Na 2 CO 3 mol Na 2 CO 3 The solution is prepared by dissolving 53 g of Na 2 CO 3 in water and diluting to 5 L.

Example: Calculating the Molarity using different algebraic relationships How would you prepare 50 m. L portions of standard solutions that are 0. 005 M, 0. 002 M, and 0. 001 M in a standard 0. 01 M Na+? To solve this the relationship Vconcd x cconcd = Vdil x cdil Vconcd = Vdil x cdil = 50 m. L x 0. 005 mmol Na+ /m. L = 25 m. L cconcd 0. 01 mmol Na+ /m. L To produce 50 m. L of 0. 005 M Na+, 25 m. L of the concentration solution should be diluted to 50 m. L.

How to deal with titration data… The following two examples show the two types of volumetric calculations. The first involves computing the molarity of solutions that have been standardized against either a primary standard or another standard solution. The second example involves calculating the amount of analyte in a sample from titration data.

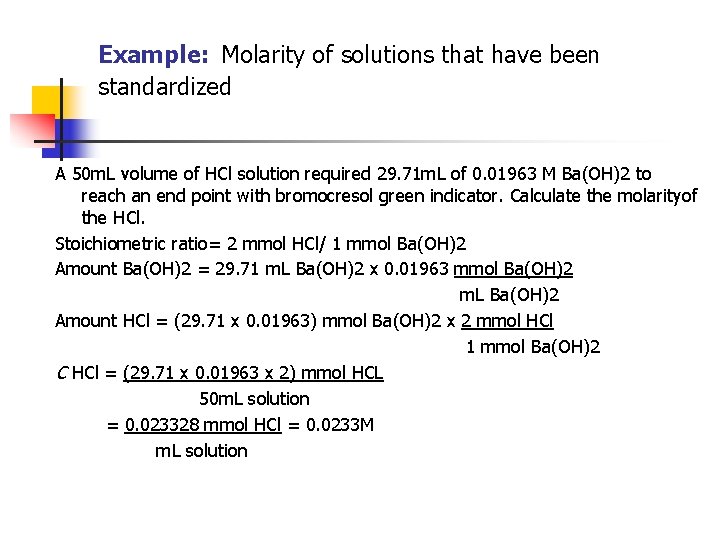
Example: Molarity of solutions that have been standardized A 50 m. L volume of HCl solution required 29. 71 m. L of 0. 01963 M Ba(OH)2 to reach an end point with bromocresol green indicator. Calculate the molarityof the HCl. Stoichiometric ratio= 2 mmol HCl/ 1 mmol Ba(OH)2 Amount Ba(OH)2 = 29. 71 m. L Ba(OH)2 x 0. 01963 mmol Ba(OH)2 m. L Ba(OH)2 Amount HCl = (29. 71 x 0. 01963) mmol Ba(OH)2 x 2 mmol HCl 1 mmol Ba(OH)2 C HCl = (29. 71 x 0. 01963 x 2) mmol HCL 50 m. L solution = 0. 023328 mmol HCl = 0. 0233 M m. L solution

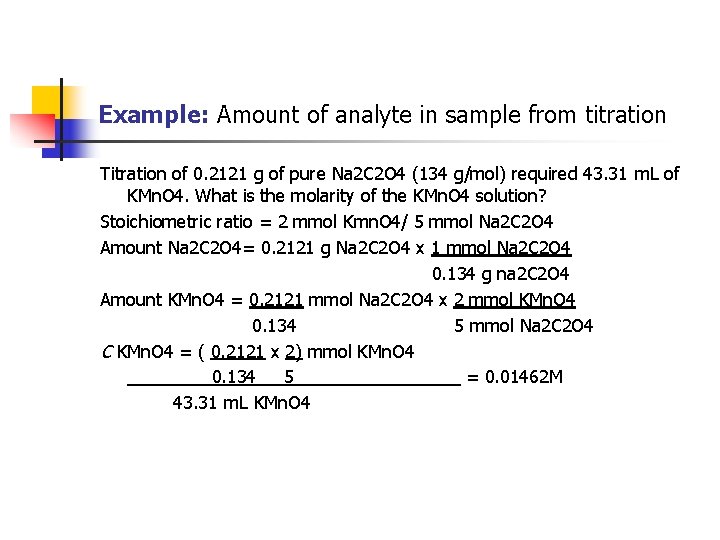
Example: Amount of analyte in sample from titration Titration of 0. 2121 g of pure Na 2 C 2 O 4 (134 g/mol) required 43. 31 m. L of KMn. O 4. What is the molarity of the KMn. O 4 solution? Stoichiometric ratio = 2 mmol Kmn. O 4/ 5 mmol Na 2 C 2 O 4 Amount Na 2 C 2 O 4= 0. 2121 g Na 2 C 2 O 4 x 1 mmol Na 2 C 2 O 4 0. 134 g na 2 C 2 O 4 Amount KMn. O 4 = 0. 2121 mmol Na 2 C 2 O 4 x 2 mmol KMn. O 4 0. 134 5 mmol Na 2 C 2 O 4 C KMn. O 4 = ( 0. 2121 x 2) mmol KMn. O 4 0. 134 5 = 0. 01462 M 43. 31 m. L KMn. O 4

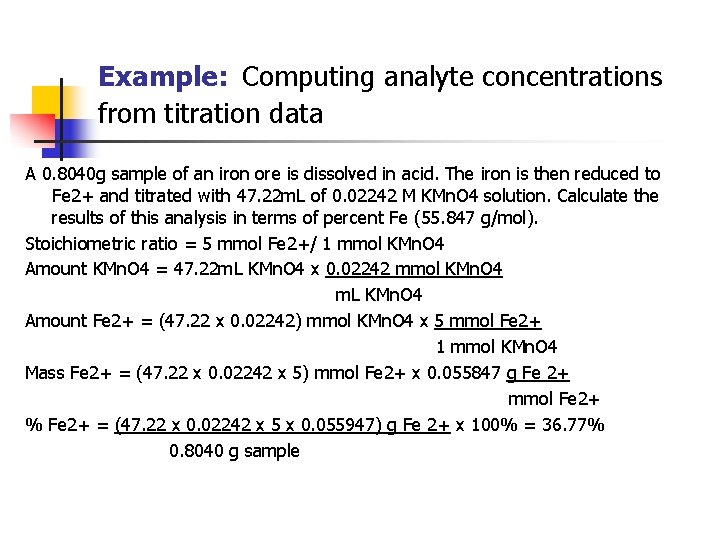
Example: Computing analyte concentrations from titration data A 0. 8040 g sample of an iron ore is dissolved in acid. The iron is then reduced to Fe 2+ and titrated with 47. 22 m. L of 0. 02242 M KMn. O 4 solution. Calculate the results of this analysis in terms of percent Fe (55. 847 g/mol). Stoichiometric ratio = 5 mmol Fe 2+/ 1 mmol KMn. O 4 Amount KMn. O 4 = 47. 22 m. L KMn. O 4 x 0. 02242 mmol KMn. O 4 m. L KMn. O 4 Amount Fe 2+ = (47. 22 x 0. 02242) mmol KMn. O 4 x 5 mmol Fe 2+ 1 mmol KMn. O 4 Mass Fe 2+ = (47. 22 x 0. 02242 x 5) mmol Fe 2+ x 0. 055847 g Fe 2+ mmol Fe 2+ % Fe 2+ = (47. 22 x 0. 02242 x 5 x 0. 055947) g Fe 2+ x 100% = 36. 77% 0. 8040 g sample

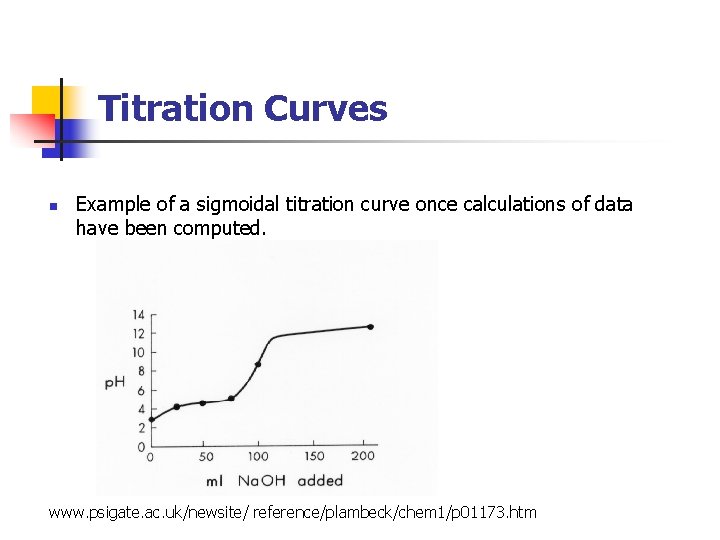
Titration Curves n Example of a sigmoidal titration curve once calculations of data have been computed. www. psigate. ac. uk/newsite/ reference/plambeck/chem 1/p 01173. htm

References n n Skoog, D. , West, D. , Holler, F. J. , & Crouch, S. (2000). Analytical Chemistry: An Introduction. 7 th ed. Thomson Learning, Inc: United States of America. http: //wine 1. sb. fsu. edu/chm 1045/notes/Aque ous/Stoich/Aqua 02. htm www. psigate. ac. uk/newsite/ reference/plambeck/chem 1/p 01173. htm http: //www 2. hmc. edu/~karukstis/chem 21 f 20 01/tutorials/tutorial. Stoichi. Frame. html
 Complexometric titration definition
Complexometric titration definition Types of titrations
Types of titrations Titration vs back titration
Titration vs back titration Example of auxiliary complexing agent
Example of auxiliary complexing agent Volhard titration calculation example
Volhard titration calculation example Buffers and titrations
Buffers and titrations Neutralization titrations
Neutralization titrations What is the titration formula?
What is the titration formula? Good titrations
Good titrations Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Redox reaction example
Redox reaction example Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Maribel is taking advantage of the sale
Maribel is taking advantage of the sale Homophones are words that
Homophones are words that Ppf
Ppf Actual mechanical advantage vs ideal mechanical advantage
Actual mechanical advantage vs ideal mechanical advantage What is stoichiometric
What is stoichiometric