Titrations Main Idea Titrations are an application of







- Slides: 21

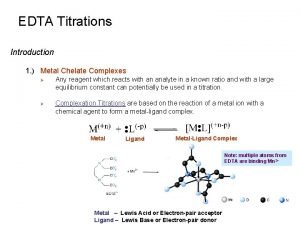
Titrations Main Idea: Titrations are an application of acid-base neutralization reactions that require the use of an indicator. 1

Stoichiometry • The stoichiometry of an acid-base neutralization reaction is the same as that of any other reaction that occurs in solution (they are double displacement reactions, after all). • For example, in the reaction of sodium hydroxide and hydrogen chloride, 1 mol of Na. OH neutralizes 1 mol of HCl: Na. OH (aq) + HCl (aq) Na. Cl (aq) + H 2 O (l) • Stoichiometry provides the basis for a procedure called titration, which is used to determine the concentrations of acidic and basic solutions. 2

Titration • Titration is a method for determining the concentration of a solution by reacting a known volume of that solution with a solution of known concentration. • If you wish to find the concentration of an acid solution, you would titrate the acid solution with a solution of a base of known concentration. • You could also titrate a base of unknown concentration with an acid of known concentration. 3

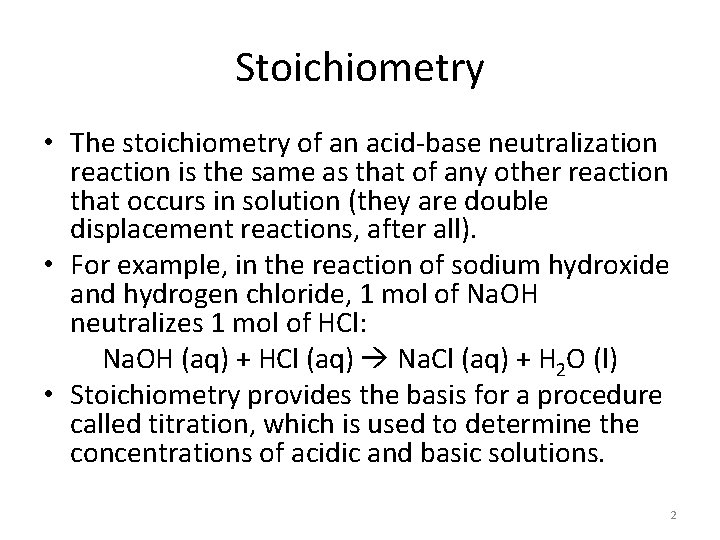
In the titration of an acid by a base, the p. H meter measures the p. H of the acid solution in the beaker as a solution of a base with a known concentration is added from the buret. http: //wps. prenhall. com/wps/media/objects/3 312/3392202/blb 1703. html 4

How is an acid-base titration performed? • The figure on the previous slide illustrates one type of setup for the titration procedure outlined on the next slide. • In the procedure pictured on Slide 4, a p. H meter is used to monitor the change in p. H as the titration progresses. 5

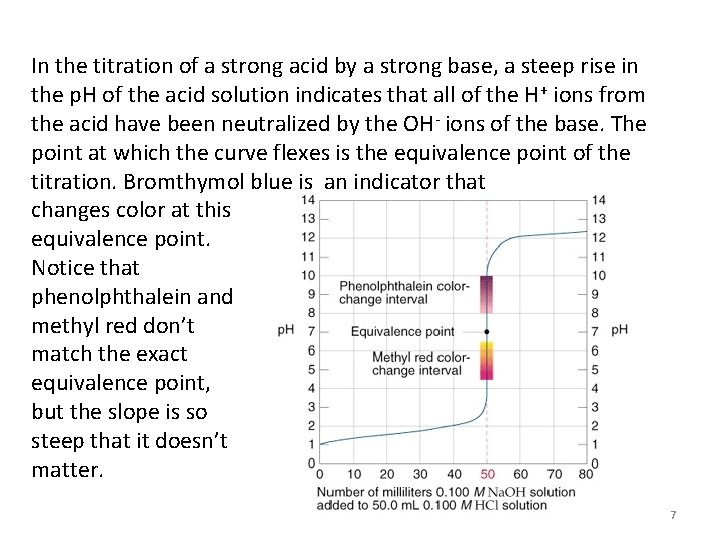
Titration Procedure 1) A measured volume of an acidic or basic solution of unknown concentration is placed in a beaker. The electrodes of a p. H meter are immersed in this solution, and the initial p. H of the solution is read and recorded. 2) A buret is filled with the titrating solution of known concentration. This is called the standard solution, or titrant. 3) Measured volumes of the standard solution are added slowly and mixed into the solution in the beaker. The p. H is read and recorded after each addition. This process continues until the reaction reaches the equivalence point, which is the point at which moles of H+ ion from the acid equal moles of OH- ion from the base. 6

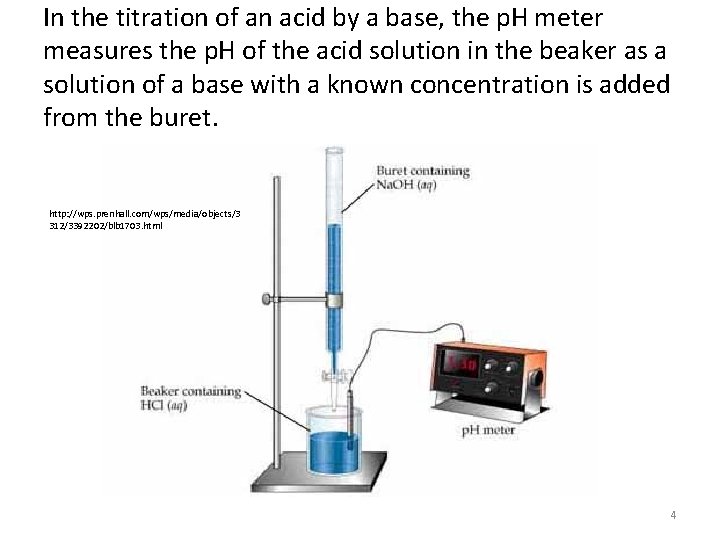
In the titration of a strong acid by a strong base, a steep rise in the p. H of the acid solution indicates that all of the H+ ions from the acid have been neutralized by the OH- ions of the base. The point at which the curve flexes is the equivalence point of the titration. Bromthymol blue is an indicator that changes color at this equivalence point. Notice that phenolphthalein and methyl red don’t match the exact equivalence point, but the slope is so steep that it doesn’t matter. 7

Strong-Strong Titration • The previous slide shows how the p. H of the solution changes during the titration of 50. 0 m. L of 0. 100 M HCl, a strong acid with 0. 100 M Na. OH, a strong base. • The inital p. H of the 0. 100 M HCl is 1. 00. – As Na. OH is added, the acid is neutralized and the solution’s p. H increases gradually. – When nearly all of the H+ ions from the acid have been used up, the p. H increases dramatically with the addition of an exceedingly small volume of Na. OH. – This abrupt change in p. H occurs at the equivalence point of the titration. – Beyond the equivalence point, the addition of more Na. OH again results in the gradual increase in p. H. 8

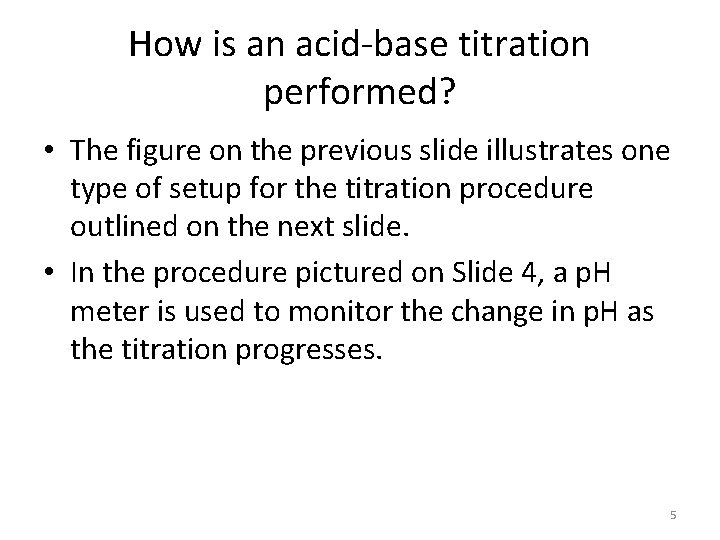
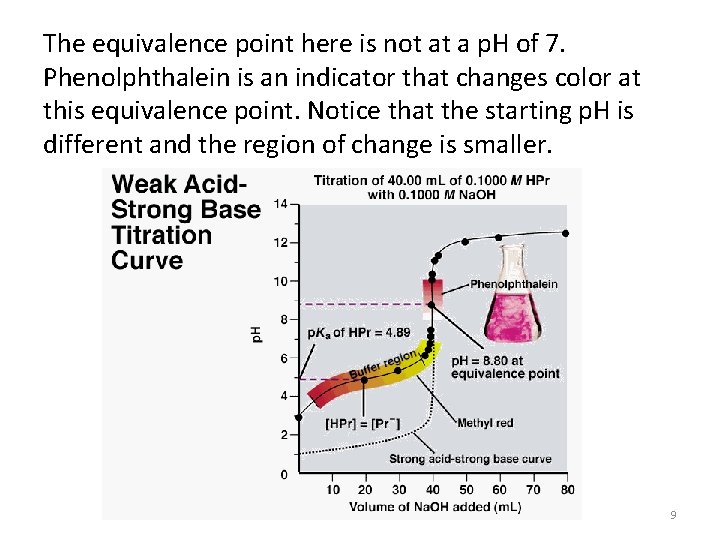
The equivalence point here is not at a p. H of 7. Phenolphthalein is an indicator that changes color at this equivalence point. Notice that the starting p. H is different and the region of change is smaller. 9

Strong-Weak Titrations • You might think that all titrations must have an equivalence point at p. H 7 because that is the point at which concentrations of hydrogen ions and hydroxide ions are equal and the solution is neutral. • This is not the case – some titrations have equivalence points at p. H values less than 7, and some have equivalence points at p. H values greater than 7. • These differences occur because of reactions between the newly formed salts and water – salt hydrolysis. – Some salts are basic (weak acid, strong base) and some salts are acidic (strong acid, weak base). • The previous slide shows that the equivalence point for the titration of HPr (a weak acid) with Na. OH (a strong base) lies at p. H 8. 80. 10

Acid-Base Indicators • Chemists often use a chemical dye rather than a p. H meter to detect the equivalence point of an acid-base titration. • Chemical dyes whose colors are affected by acidic and basic solutions are called acid-base indicators. • Many natural substances act as indicators. – If you use lemon juice in your tea, you might have noticed that the brown color of tea gets lighter when lemon juice is added. – Tea contains compounds called polyphenols that have slightly ionizable hydrogen atoms and therefore are weak acids. – Adding acid in the form of lemon juice to a cup of tea lessens the degree of ionization, and the color of the un-ionized polyphenols becomes more apparent. • Chemists have several choices in selecting indicators. – Bromthymol blue is a good choice for the titration of a strong acid with a strong base, and phenolphthalein changes color at the equivalence point of a titration of a weak acid with a strong base. 11

Indicators and Titration End Point • Many indicators used for titrations are weak acids. – Each has its own particular p. H or p. H ranges over which it changes color. • The point at which the indicator used in a titration changes color is called the end point of the titration. – It is important to choose an indicator for a titration that will change color at the equivalence point of the titration. – Remember that the role of the indicator is to indicate to you, by means of a color change, that just enough of the titrating solution has been added to neutralize the unknown solution. • Equivalence point ≠ End point! – BUT for strong-strong titrations, the p. H change is so steep and so large, that the are approximately equal. 12

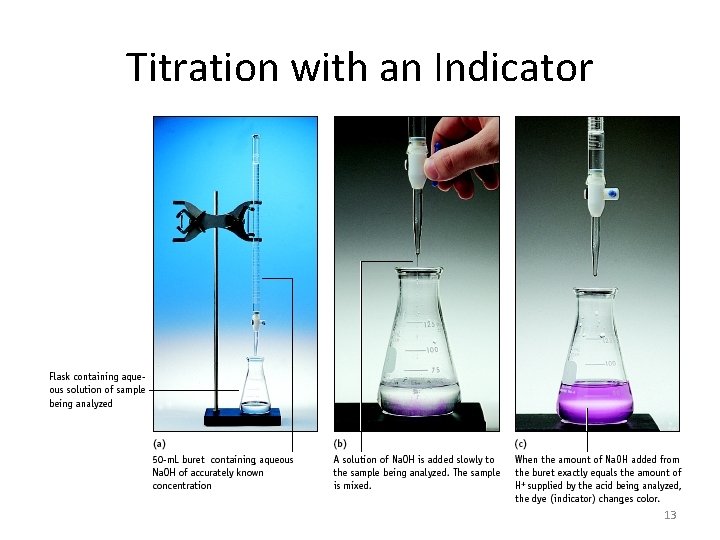
Titration with an Indicator 13

What’s the Point of a Titration Again? • To find the unknown concentration of an acid or a base. • So you perform the actual titration noting the volume you started with and how much volume of the titrant you added and then. . . • Math! (Oh no! Not math! Anything but math!) 14

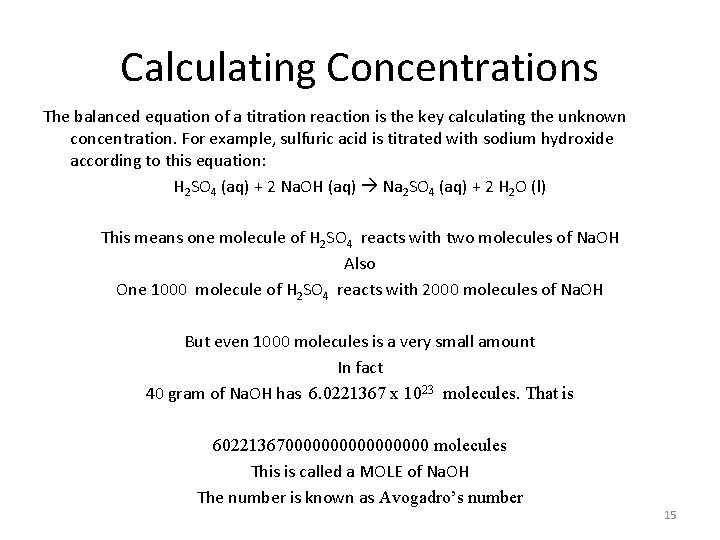
Calculating Concentrations The balanced equation of a titration reaction is the key calculating the unknown concentration. For example, sulfuric acid is titrated with sodium hydroxide according to this equation: H 2 SO 4 (aq) + 2 Na. OH (aq) Na 2 SO 4 (aq) + 2 H 2 O (l) This means one molecule of H 2 SO 4 reacts with two molecules of Na. OH Also One 1000 molecule of H 2 SO 4 reacts with 2000 molecules of Na. OH But even 1000 molecules is a very small amount In fact 40 gram of Na. OH has 6. 0221367 x 1023 molecules. That is 6022136700000000 molecules This is called a MOLE of Na. OH The number is known as Avogadro’s number 15

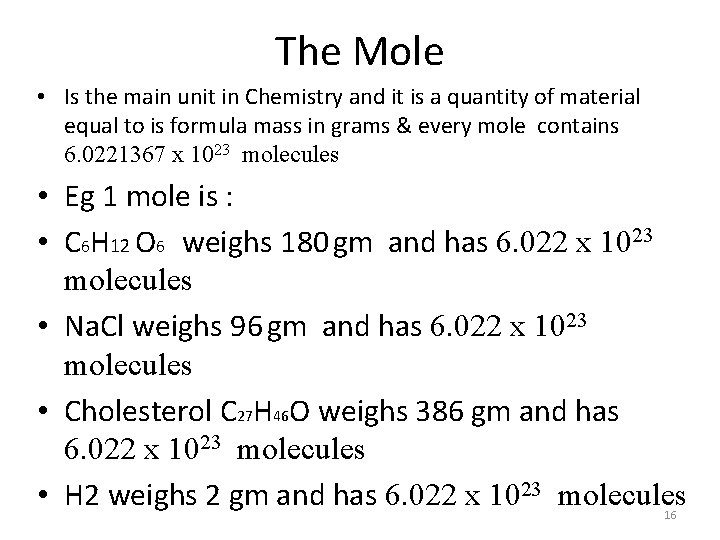
The Mole • Is the main unit in Chemistry and it is a quantity of material equal to is formula mass in grams & every mole contains 6. 0221367 x 1023 molecules • Eg 1 mole is : • C 6 H 12 O 6 weighs 180 gm and has 6. 022 x 1023 molecules • Na. Cl weighs 96 gm and has 6. 022 x 1023 molecules • Cholesterol C 27 H 46 O weighs 386 gm and has 6. 022 x 1023 molecules • H 2 weighs 2 gm and has 6. 022 x 1023 molecules 16

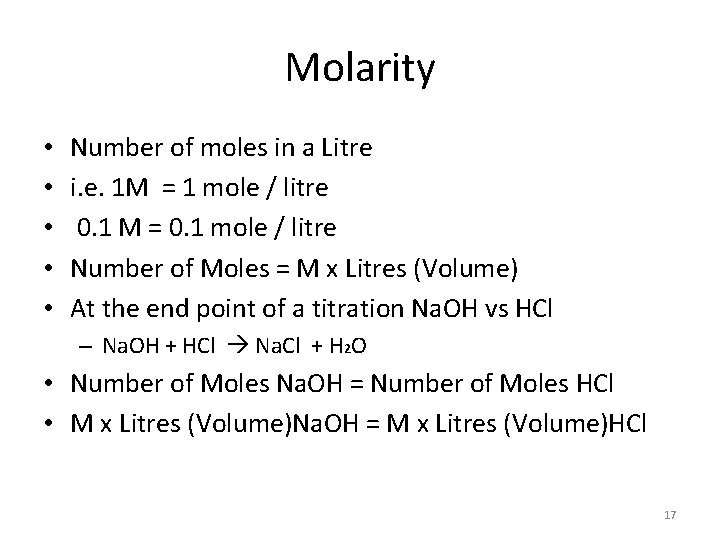
Molarity • • • Number of moles in a Litre i. e. 1 M = 1 mole / litre 0. 1 M = 0. 1 mole / litre Number of Moles = M x Litres (Volume) At the end point of a titration Na. OH vs HCl – Na. OH + HCl Na. Cl + H 2 O • Number of Moles Na. OH = Number of Moles HCl • M x Litres (Volume)Na. OH = M x Litres (Volume)HCl 17

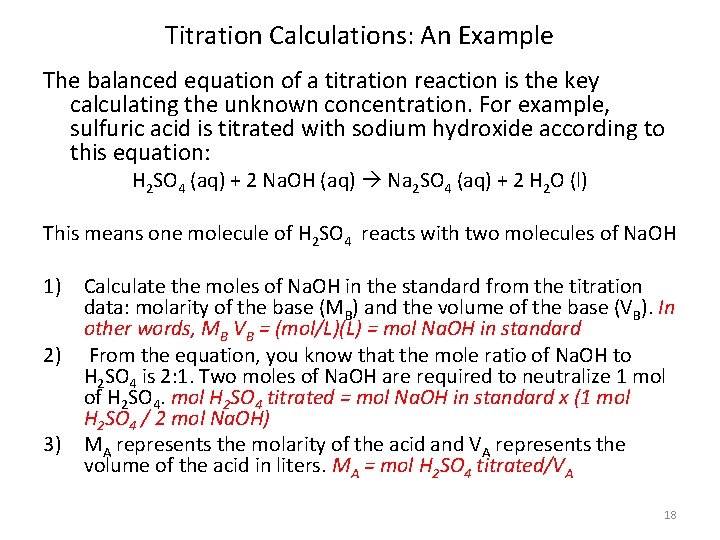
Titration Calculations: An Example The balanced equation of a titration reaction is the key calculating the unknown concentration. For example, sulfuric acid is titrated with sodium hydroxide according to this equation: H 2 SO 4 (aq) + 2 Na. OH (aq) Na 2 SO 4 (aq) + 2 H 2 O (l) This means one molecule of H 2 SO 4 reacts with two molecules of Na. OH 1) Calculate the moles of Na. OH in the standard from the titration data: molarity of the base (MB) and the volume of the base (VB). In other words, MB VB = (mol/L)(L) = mol Na. OH in standard 2) From the equation, you know that the mole ratio of Na. OH to H 2 SO 4 is 2: 1. Two moles of Na. OH are required to neutralize 1 mol of H 2 SO 4. mol H 2 SO 4 titrated = mol Na. OH in standard x (1 mol H 2 SO 4 / 2 mol Na. OH) 3) MA represents the molarity of the acid and VA represents the volume of the acid in liters. MA = mol H 2 SO 4 titrated/VA 18

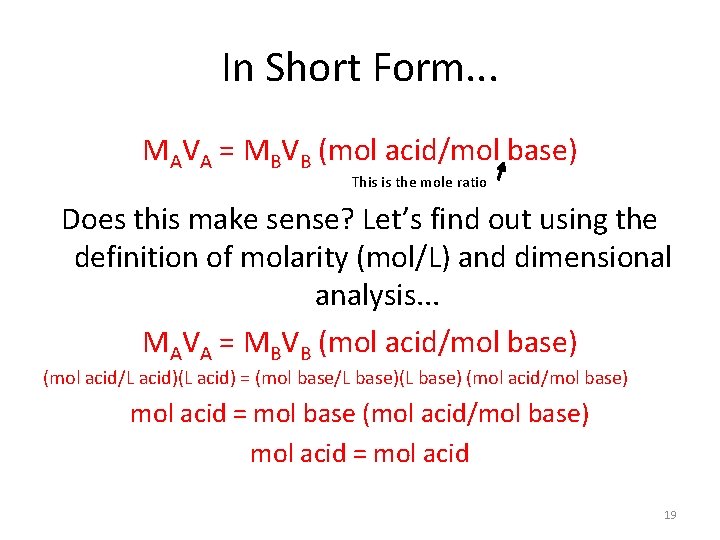
In Short Form. . . MAVA = MBVB (mol acid/mol base) This is the mole ratio Does this make sense? Let’s find out using the definition of molarity (mol/L) and dimensional analysis. . . MAVA = MBVB (mol acid/mol base) (mol acid/L acid)(L acid) = (mol base/L base)(L base) (mol acid/mol base) mol acid = mol base (mol acid/mol base) mol acid = mol acid 19

HOMEWORK QUESTIONS 1) What is the purpose of a titration? How is it performed (in general)? 2) What is the difference between the equivalence point and the end point of a titration? 3) Describe the differences in the titration curve of a strong-strong titration vs. a strong-weak titration. 4) When is the equivalence point of a titration not at p. H 7? Why does this occur? 20

MORE HOMEWORK 5) What is the molarity of a nitric acid solution if 43. 33 m. L of 0. 1000 M KOH solution is needed to neutralize 20. 00 m. L of the acid solution? 6) What is the concentration of a household ammonia cleaning solution if 49. 90 m. L of 0. 5900 M HCl is required to neutralize 25. 00 m. L of the solution? 7) How many milliliters of 0. 500 M Na. OH would neutralize 25. 00 m. L of 0. 100 M H 2 SO 4? 21
 Mikael ferm
Mikael ferm Which is the implied main idea in the text
Which is the implied main idea in the text Central idea/main idea
Central idea/main idea Contoh topic dan controlling idea
Contoh topic dan controlling idea Is the main idea the theme
Is the main idea the theme Irrelevant
Irrelevant Types of titrations
Types of titrations Types of titrations
Types of titrations Titration vs back titration
Titration vs back titration Slidetodoc.com
Slidetodoc.com Precipitation titrations
Precipitation titrations Weak acid strong base buffer
Weak acid strong base buffer Neutralization titrations
Neutralization titrations Formula of titration
Formula of titration Good titrations
Good titrations Expository preaching vs. exegetical preaching
Expository preaching vs. exegetical preaching Walau bagaimanapun penanda wacana
Walau bagaimanapun penanda wacana What is the topic sentence? *
What is the topic sentence? * Two types of main idea
Two types of main idea Main ideaa
Main ideaa Thesis statement title
Thesis statement title Topic vs theme
Topic vs theme