Chapter 1 cont Fluid Their Properties WHAT IS








- Slides: 38

Chapter 1 (cont. ) Fluid & Their Properties

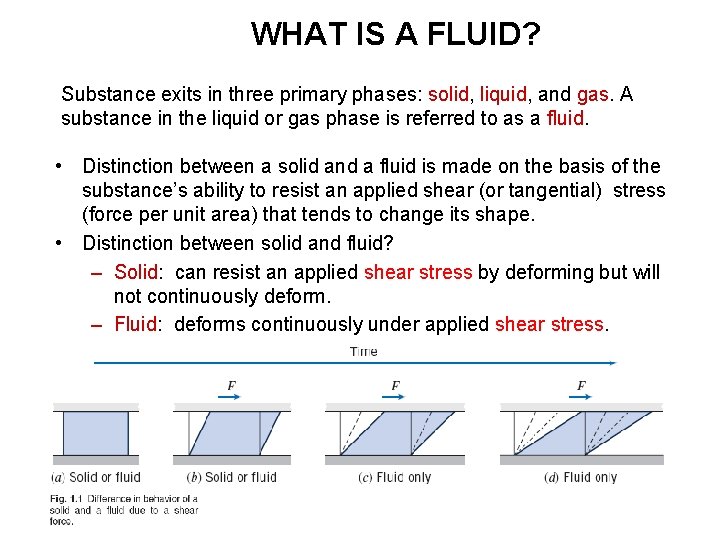
WHAT IS A FLUID? Substance exits in three primary phases: solid, liquid, and gas. A substance in the liquid or gas phase is referred to as a fluid. • Distinction between a solid and a fluid is made on the basis of the substance’s ability to resist an applied shear (or tangential) stress (force per unit area) that tends to change its shape. • Distinction between solid and fluid? – Solid: can resist an applied shear stress by deforming but will not continuously deform. – Fluid: deforms continuously under applied shear stress.

WHAT IS A FLUID? Stress is defined as force per unit area and is determined by dividing the force by the area upon which it acts. The normal component of the force acting on a surface per unit area is called the normal stress, and tangential component of a force acting on a surface per unit area is called shear stress. In a fluid at rest, the normal stress is called pressure. A fluid at rest is at a state of zero shear stress. When walls are removed or a liquid container is tiled, a shear stress develops as the liquid moves to reestablish a horizontal free surface.

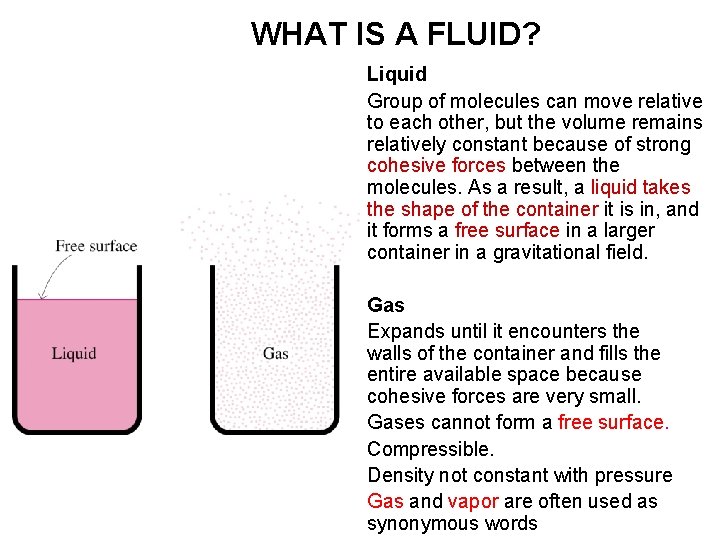
WHAT IS A FLUID? Liquid Group of molecules can move relative to each other, but the volume remains relatively constant because of strong cohesive forces between the molecules. As a result, a liquid takes the shape of the container it is in, and it forms a free surface in a larger container in a gravitational field. Gas Expands until it encounters the walls of the container and fills the entire available space because cohesive forces are very small. Gases cannot form a free surface. Compressible. Density not constant with pressure Gas and vapor are often used as synonymous words

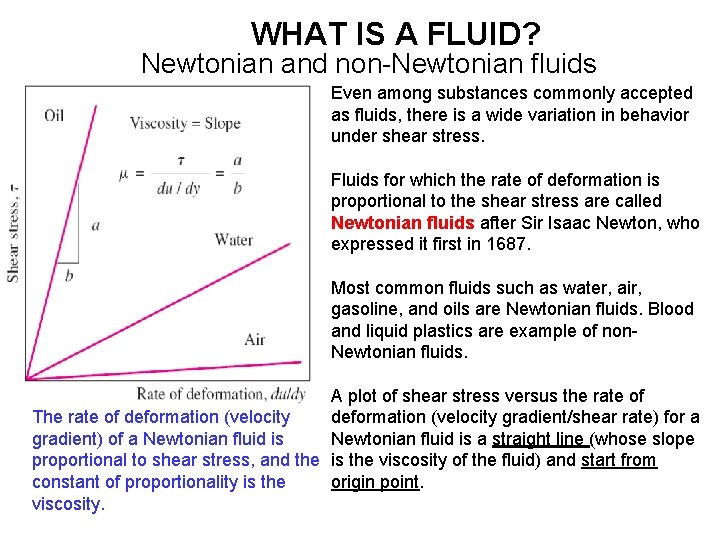
WHAT IS A FLUID? Newtonian and non-Newtonian fluids Even among substances commonly accepted as fluids, there is a wide variation in behavior under shear stress. Fluids for which the rate of deformation is proportional to the shear stress are called Newtonian fluids after Sir Isaac Newton, who expressed it first in 1687. Most common fluids such as water, air, gasoline, and oils are Newtonian fluids. Blood and liquid plastics are example of non. Newtonian fluids. A plot of shear stress versus the rate of The rate of deformation (velocity gradient/shear rate) for a gradient) of a Newtonian fluid is a straight line (whose slope proportional to shear stress, and the is the viscosity of the fluid) and start from constant of proportionality is the origin point. viscosity.

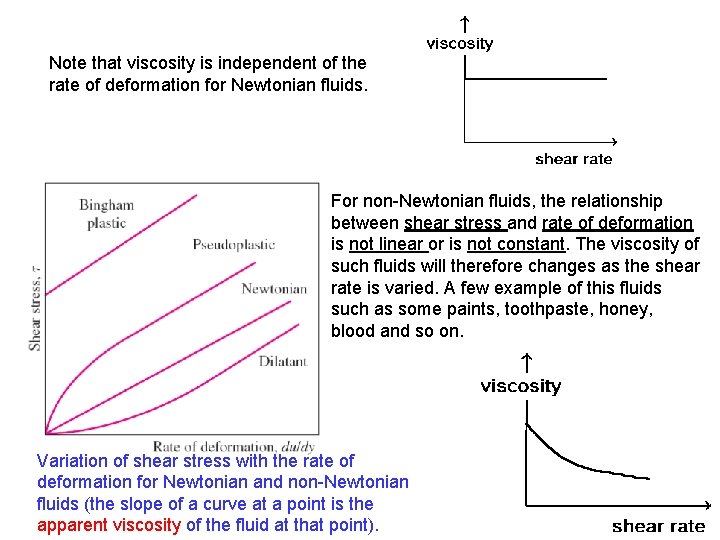
Note that viscosity is independent of the rate of deformation for Newtonian fluids. For non-Newtonian fluids, the relationship between shear stress and rate of deformation is not linear or is not constant. The viscosity of such fluids will therefore changes as the shear rate is varied. A few example of this fluids such as some paints, toothpaste, honey, blood and so on. Variation of shear stress with the rate of deformation for Newtonian and non-Newtonian fluids (the slope of a curve at a point is the apparent viscosity of the fluid at that point).

Density And Specific Gravity The density of a substance is the quantity of matter contained in a unit volume of the substance. It can be expressed in three different ways. 1. Mass Density • Mass Density, p, (rho), is defined as the mass of substance per unit (rho), volume. • Units: Kilograms per cubic metre, kg / m 3 (or kgm-3 ) • Typical values: Water = 1000 kgm-3 , Mercury = 13546 kgm-3 Air = 1. 23 kgm-3 , Paraffin Oil = 800 kgm-3. (at pressure =1. 013 x 10 -5 N m-2 and Temperature = 288. 15 K. ) 2. • • • Specific volume Is defined as v = 1/r = V/m. For a gas, density depends on temperature and pressure. The density gases is proportional to pressure and inversely proportional to temperature. • Unit is m 3/kg

3. Specific weight • Specific Weight , (sometimes (gamma), and sometimes known as specific (gamma) gravity) is defined as the weight per unit volume. or The force exerted by gravity, g, upon a unit volume of the substance. • The Relationship between g and can be determined by Newton’s 2 nd Law, since weight per unit volume = mass per unit volume x g = pg • Units: Newton’s per cubic metre, N/m 3(or Nm-3 ) • Typical values: Water =9814 N m-3 , Mercury = 132943 N m-3 , Air =12. 07 N m-3. 4. Specific gravity, or relative density • Is defined as the ratio of the density of a substance to the density of some standard substance at a specified temperature (usually water at 4°C), i. e. , SG=p/p. H 20 at 4°C. SG is a dimensionless quantity.

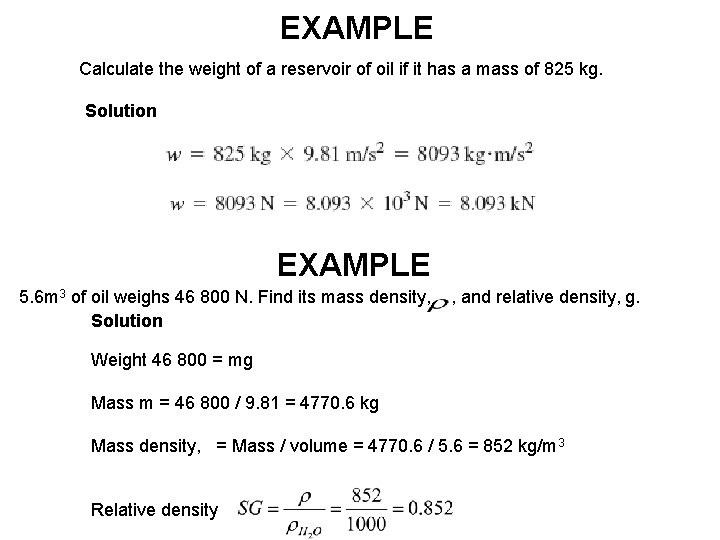
EXAMPLE Calculate the weight of a reservoir of oil if it has a mass of 825 kg. Solution EXAMPLE 5. 6 m 3 of oil weighs 46 800 N. Find its mass density, , and relative density, g. Solution Weight 46 800 = mg Mass m = 46 800 / 9. 81 = 4770. 6 kg Mass density, = Mass / volume = 4770. 6 / 5. 6 = 852 kg/m 3 Relative density

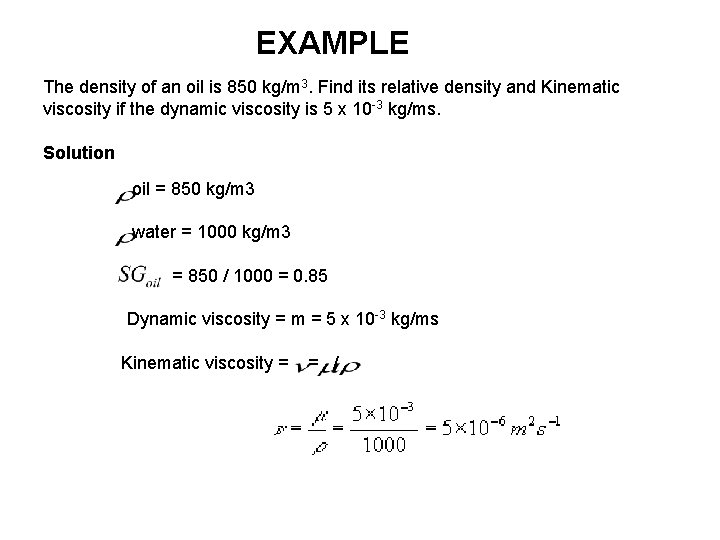
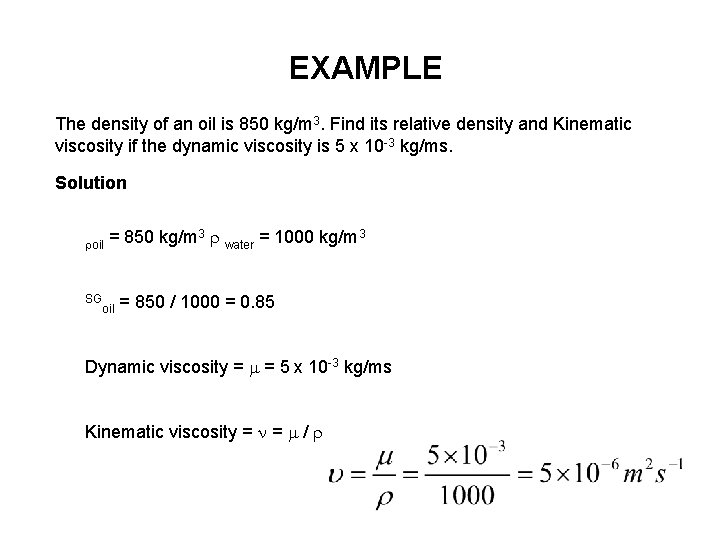
EXAMPLE The density of an oil is 850 kg/m 3. Find its relative density and Kinematic viscosity if the dynamic viscosity is 5 x 10 -3 kg/ms. Solution oil = 850 kg/m 3 water = 1000 kg/m 3 = 850 / 1000 = 0. 85 Dynamic viscosity = m = 5 x 10 -3 kg/ms Kinematic viscosity = = /

Density of Ideal Gases • Equation of State: equation for the relationship between pressure, temperature, and density. • The simplest and best-known equation of state is the ideal-gas equation. or • where P is the absolute pressure, v is the specific volume, T is thermodynamic (absolute) temperature, and R is the constant. The value of R for several substance is given in Table A-1. • Ideal-gas equation holds for most gases. • However, dense gases such as water vapor and refrigerant vapor should not be treated as ideal gases. Tables should be consulted for their properties, e. g. , Tables A-4 in textbook.

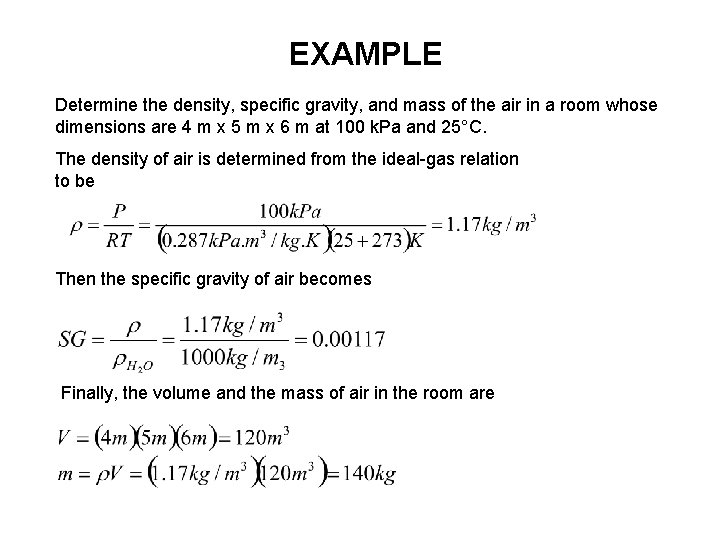
EXAMPLE Determine the density, specific gravity, and mass of the air in a room whose dimensions are 4 m x 5 m x 6 m at 100 k. Pa and 25°C. The density of air is determined from the ideal-gas relation to be Then the specific gravity of air becomes Finally, the volume and the mass of air in the room are

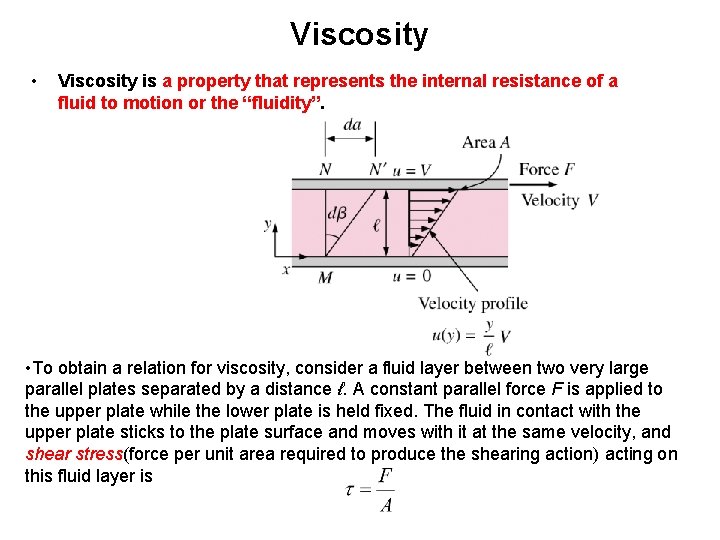
Viscosity • Viscosity is a property that represents the internal resistance of a fluid to motion or the “fluidity”. • To obtain a relation for viscosity, consider a fluid layer between two very large parallel plates separated by a distance ℓ. A constant parallel force F is applied to the upper plate while the lower plate is held fixed. The fluid in contact with the upper plate sticks to the plate surface and moves with it at the same velocity, and shear stress(force per unit area required to produce the shearing action) acting on this fluid layer is

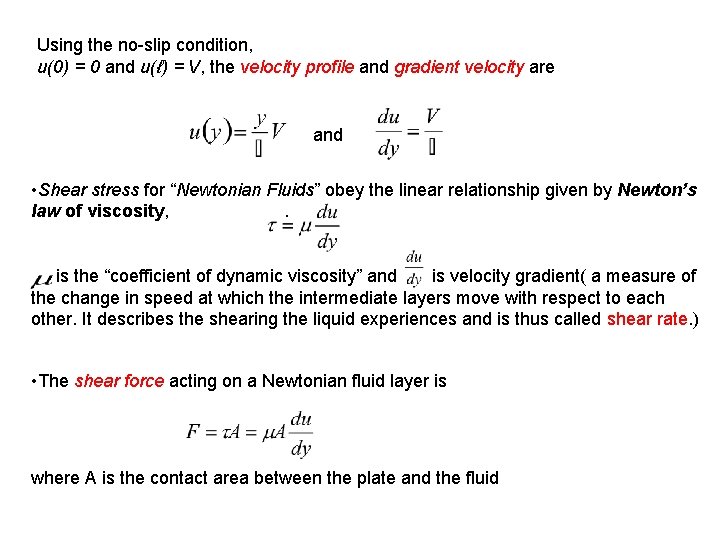
Using the no-slip condition, u(0) = 0 and u(ℓ) = V, the velocity profile and gradient velocity are and • Shear stress for “Newtonian Fluids” obey the linear relationship given by Newton’s law of viscosity, . is the “coefficient of dynamic viscosity” and is velocity gradient( a measure of the change in speed at which the intermediate layers move with respect to each other. It describes the shearing the liquid experiences and is thus called shear rate. ) • The shear force acting on a Newtonian fluid layer is where A is the contact area between the plate and the fluid

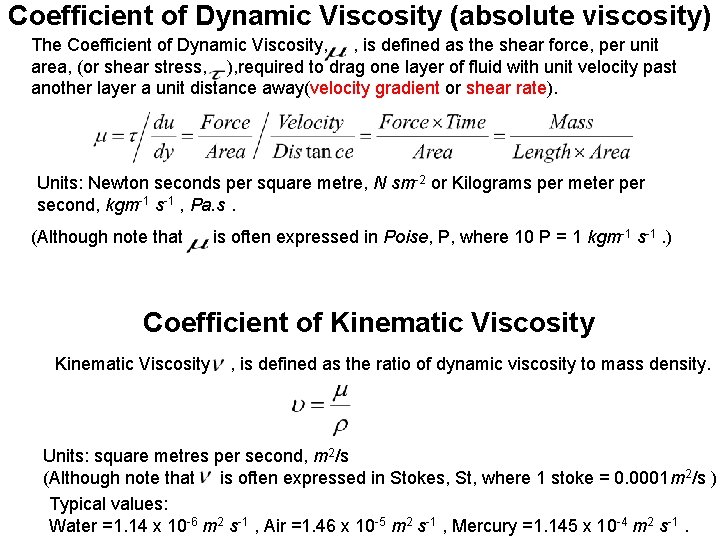
Coefficient of Dynamic Viscosity (absolute viscosity) The Coefficient of Dynamic Viscosity, , is defined as the shear force, per unit area, (or shear stress, ), required to drag one layer of fluid with unit velocity past another layer a unit distance away(velocity gradient or shear rate). Units: Newton seconds per square metre, N sm-2 or Kilograms per meter per second, kgm-1 s-1 , Pa. s. (Although note that is often expressed in Poise, P, where 10 P = 1 kgm-1 s-1. ) Coefficient of Kinematic Viscosity, , is defined as the ratio of dynamic viscosity to mass density. Units: square metres per second, m 2/s (Although note that is often expressed in Stokes, St, where 1 stoke = 0. 0001 m 2/s ) Typical values: Water =1. 14 x 10 -6 m 2 s-1 , Air =1. 46 x 10 -5 m 2 s-1 , Mercury =1. 145 x 10 -4 m 2 s-1.

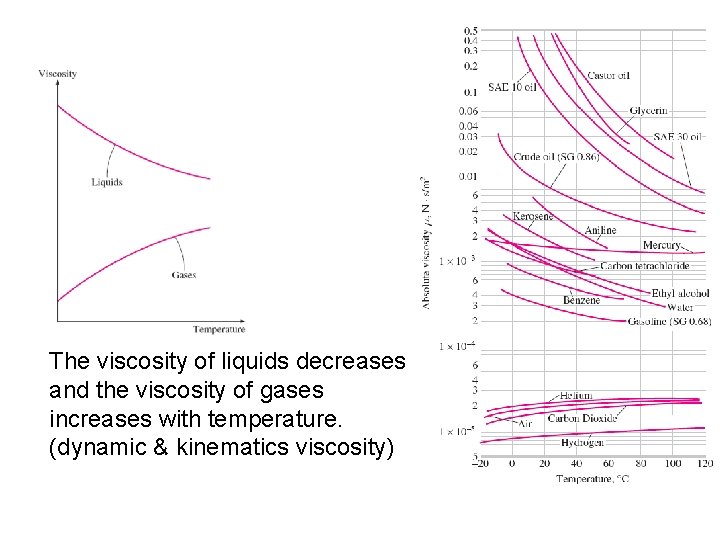
More About Viscosity • Viscosity of a fluid depends on both temperature and pressure, although the dependence on pressure rather weak. • For liquids, both dynamic and kinematic viscosities independent of pressure. • For gases, dynamic viscosity is independent of pressure but no for kinematic viscosity since the density of a gas is proportional to its pressure. • Viscosity of a fluid is a measure of its “resistance to deformation”. • Viscosity is due to the internal frictional force that develops between different layers of fluids as they are forced to move relative to each other. • Viscosity is caused by the cohesive force between the molecules in liquid and by the molecular collision in gases. • Liquid decrease with temperature-because in a liquid the molecules possess more energy at higher temperature, and they can oppose the large cohesive intermolecular force more strongly. As a result, the energized liquid molecules can move freely. • In a gas-the intermolecular forces are negligible, and gas molecules at high temperature move randomly at higher velocities. As a result, more molecular collisions per unit volume per unit time and therefore in greater resistance to flow

The viscosity of liquids decreases and the viscosity of gases increases with temperature. (dynamic & kinematics viscosity)

EXAMPLE The density of an oil is 850 kg/m 3. Find its relative density and Kinematic viscosity if the dynamic viscosity is 5 x 10 -3 kg/ms. Solution roil = 850 kg/m 3 r water = 1000 kg/m 3 SG = 850 / 1000 = 0. 85 oil Dynamic viscosity = m = 5 x 10 -3 kg/ms Kinematic viscosity = n = m / r

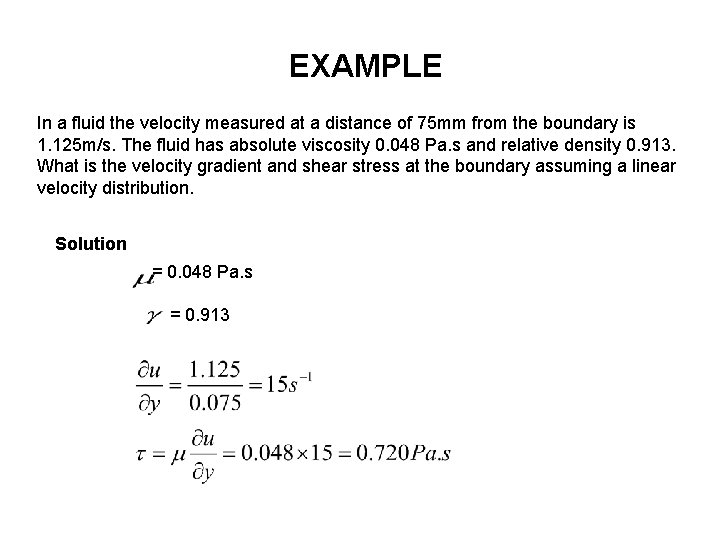
EXAMPLE In a fluid the velocity measured at a distance of 75 mm from the boundary is 1. 125 m/s. The fluid has absolute viscosity 0. 048 Pa. s and relative density 0. 913. What is the velocity gradient and shear stress at the boundary assuming a linear velocity distribution. Solution = 0. 048 Pa. s = 0. 913

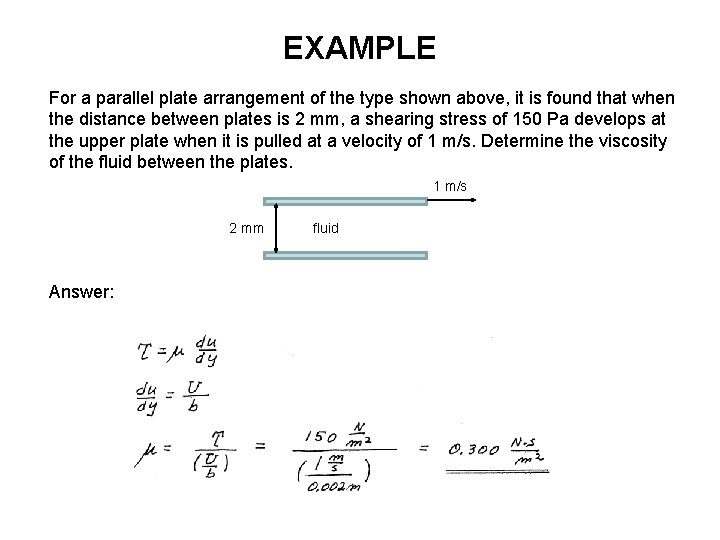
EXAMPLE For a parallel plate arrangement of the type shown above, it is found that when the distance between plates is 2 mm, a shearing stress of 150 Pa develops at the upper plate when it is pulled at a velocity of 1 m/s. Determine the viscosity of the fluid between the plates. 1 m/s 2 mm Answer: fluid

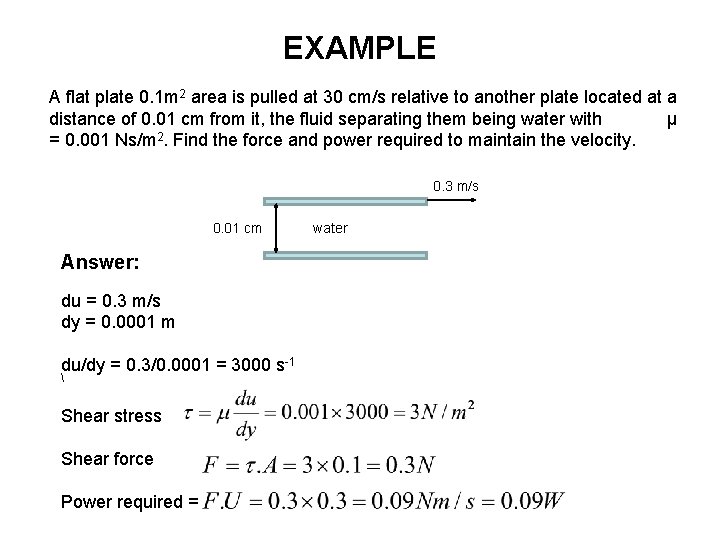
EXAMPLE A flat plate 0. 1 m 2 area is pulled at 30 cm/s relative to another plate located at a distance of 0. 01 cm from it, the fluid separating them being water with μ = 0. 001 Ns/m 2. Find the force and power required to maintain the velocity. 0. 3 m/s 0. 01 cm Answer: du = 0. 3 m/s dy = 0. 0001 m du/dy = 0. 3/0. 0001 = 3000 s-1 Shear stress Shear force Power required = water

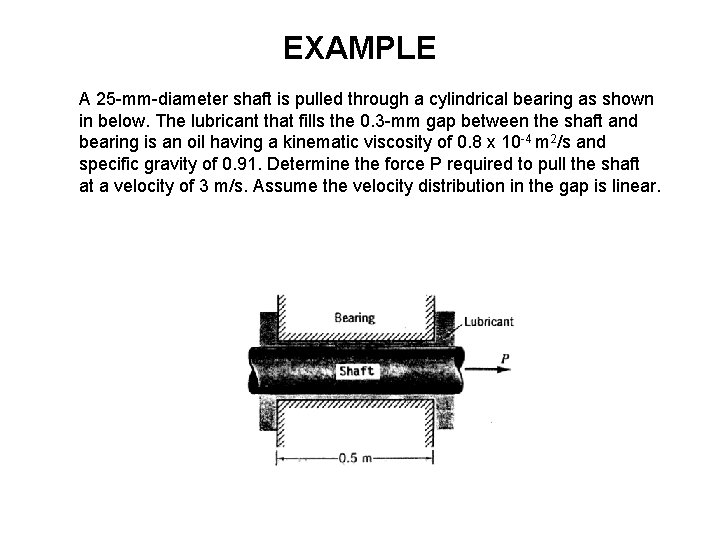
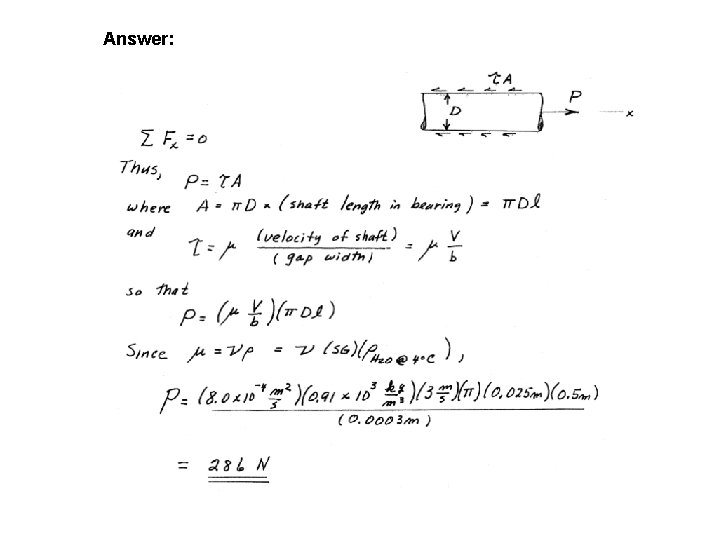
EXAMPLE A 25 -mm-diameter shaft is pulled through a cylindrical bearing as shown in below. The lubricant that fills the 0. 3 -mm gap between the shaft and bearing is an oil having a kinematic viscosity of 0. 8 x 10 -4 m 2/s and specific gravity of 0. 91. Determine the force P required to pull the shaft at a velocity of 3 m/s. Assume the velocity distribution in the gap is linear.

Answer:

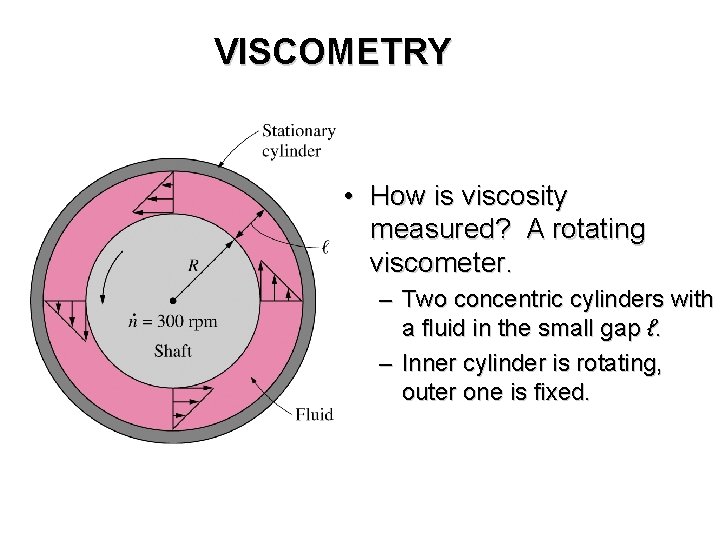
VISCOMETRY • How is viscosity measured? A rotating viscometer. – Two concentric cylinders with a fluid in the small gap ℓ. – Inner cylinder is rotating, outer one is fixed.

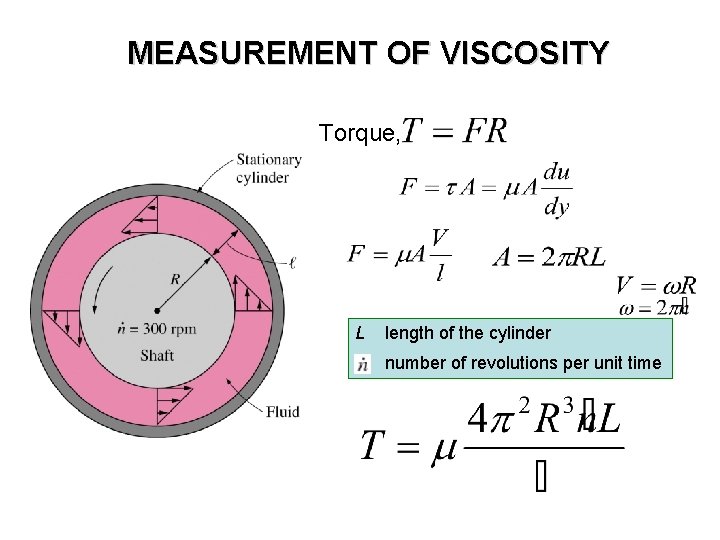
MEASUREMENT OF VISCOSITY Torque, L length of the cylinder number of revolutions per unit time

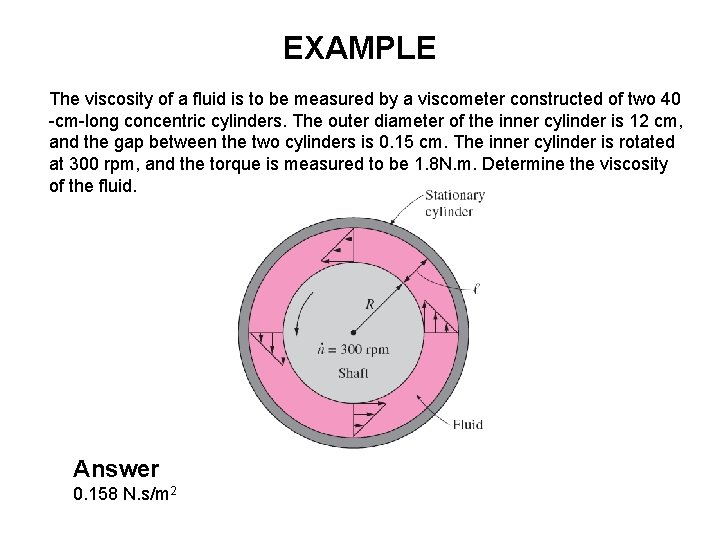
EXAMPLE The viscosity of a fluid is to be measured by a viscometer constructed of two 40 -cm-long concentric cylinders. The outer diameter of the inner cylinder is 12 cm, and the gap between the two cylinders is 0. 15 cm. The inner cylinder is rotated at 300 rpm, and the torque is measured to be 1. 8 N. m. Determine the viscosity of the fluid. Answer 0. 158 N. s/m 2

Surface Tension & Capillary Effect Examples Water droplets from rain hang from branches or leaves of trees, Water strides stay atop the liquid and paper clip(Picture)

v. Although all molecules are in constant motion, a molecule within the body of the liquid is, on average, attracted equally in all direction by other molecules surrounding it, but, at the surface between liquid and air, or the interface between one substance and another, the upward and downward attraction are unbalanced, the surface molecules being pulled inward towards the bulk of the liquid. v. This effect causes the liquid surface to behave as if it were an elastic membrane or skin under tension/stretched over the fluid mass. v. For example, a steel needle or a razor blade will float on water if placed gently on the surface because the tension developed in the hypothetical skin supports it. v. The surface tension is measured as the force acting across the unit length of a line drawn in the surface. v. It acts in the plane of the surface, normal to any line in the surface, and is the same at all points. Attractive forces acting on a liquid molecule at the surface and deep inside the liquid.

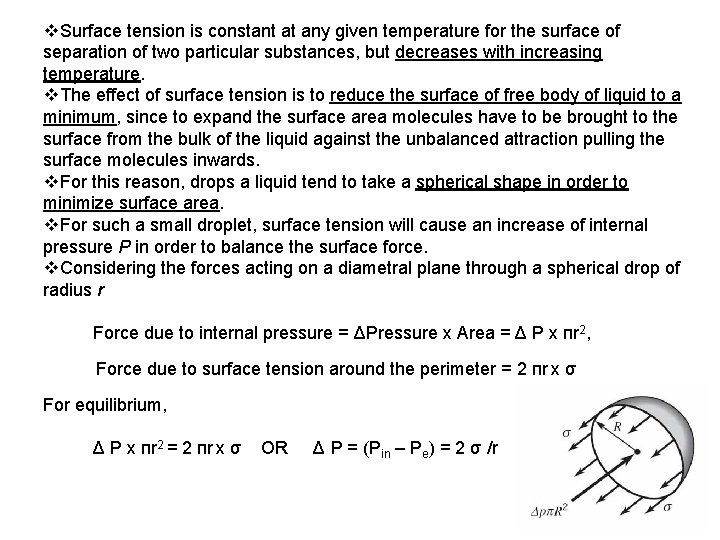
v. Surface tension is constant at any given temperature for the surface of separation of two particular substances, but decreases with increasing temperature. v. The effect of surface tension is to reduce the surface of free body of liquid to a minimum, since to expand the surface area molecules have to be brought to the surface from the bulk of the liquid against the unbalanced attraction pulling the surface molecules inwards. v. For this reason, drops a liquid tend to take a spherical shape in order to minimize surface area. v. For such a small droplet, surface tension will cause an increase of internal pressure P in order to balance the surface force. v. Considering the forces acting on a diametral plane through a spherical drop of radius r Force due to internal pressure = ΔPressure x Area = Δ P x пr 2, Force due to surface tension around the perimeter = 2 пr x σ For equilibrium, Δ P x пr 2 = 2 пr x σ OR Δ P = (Pin – Pe) = 2 σ /r

v. For small bubbles in a liquid, if this pressure is greater than the pressure of vapor or gas in a bubble, the bubble will collapse. v. In many of the problem with which engineers are concerned, the magnitude of surface tension force is very small compared with the other forces acting on the fluid and may, therefore, be neglected. v. Surface tensions forces can be reduced by the addition of detergents.

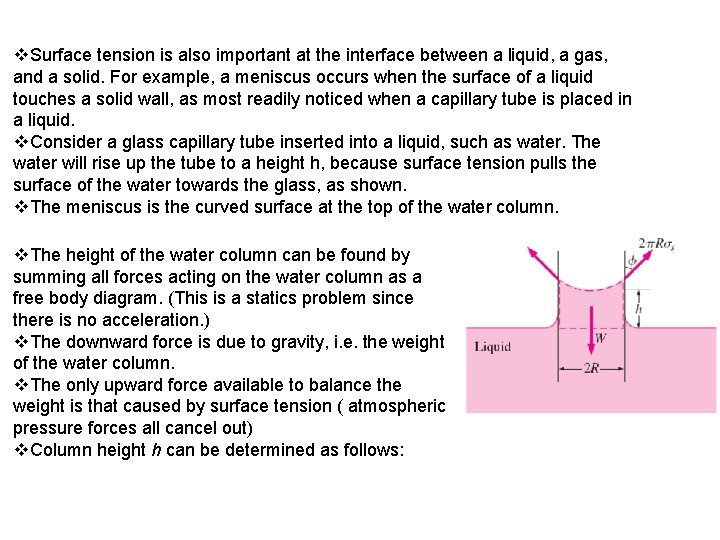
v. Surface tension is also important at the interface between a liquid, a gas, and a solid. For example, a meniscus occurs when the surface of a liquid touches a solid wall, as most readily noticed when a capillary tube is placed in a liquid. v. Consider a glass capillary tube inserted into a liquid, such as water. The water will rise up the tube to a height h, because surface tension pulls the surface of the water towards the glass, as shown. v. The meniscus is the curved surface at the top of the water column. v. The height of the water column can be found by summing all forces acting on the water column as a free body diagram. (This is a statics problem since there is no acceleration. ) v. The downward force is due to gravity, i. e. the weight of the water column. v. The only upward force available to balance the weight is that caused by surface tension ( atmospheric pressure forces all cancel out) v. Column height h can be determined as follows:

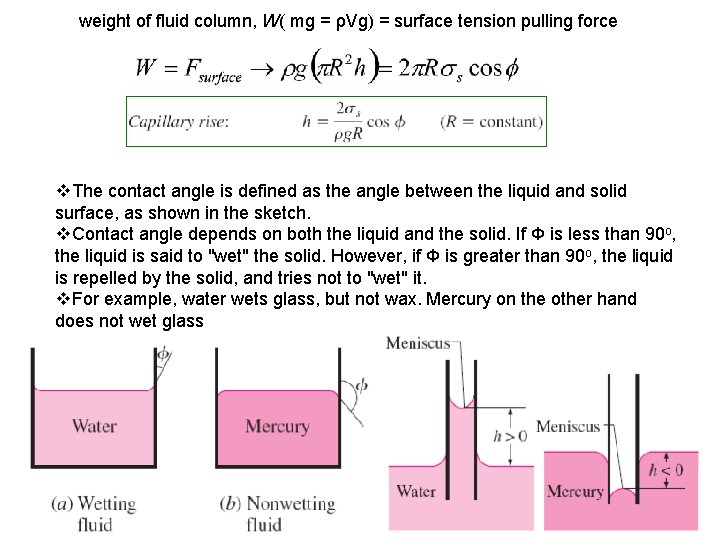
weight of fluid column, W( mg = ρVg) = surface tension pulling force v. The contact angle is defined as the angle between the liquid and solid surface, as shown in the sketch. v. Contact angle depends on both the liquid and the solid. If Ф is less than 90 o, the liquid is said to "wet" the solid. However, if Ф is greater than 90 o, the liquid is repelled by the solid, and tries not to "wet" it. v. For example, water wets glass, but not wax. Mercury on the other hand does not wet glass

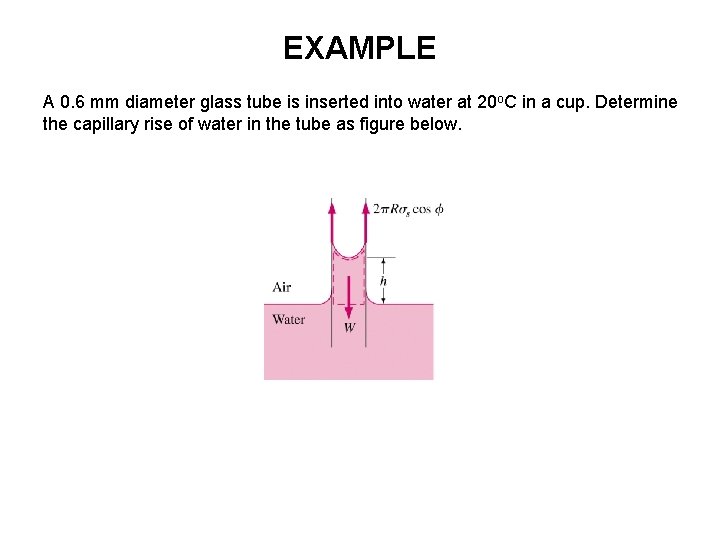
EXAMPLE A 0. 6 mm diameter glass tube is inserted into water at 20 o. C in a cup. Determine the capillary rise of water in the tube as figure below.

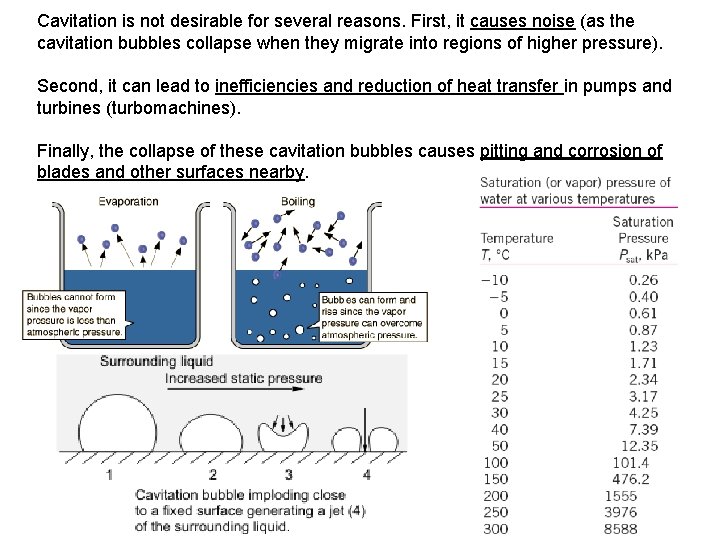
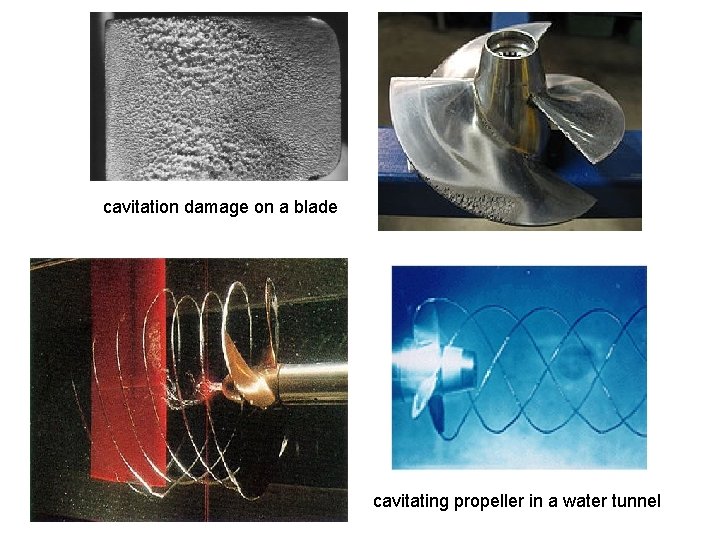
Vapor Pressure The pressure(absolute pressure) at which a liquid will boil is called its vapor pressure. This pressure is a function of temperature (vapor pressure increases with temperature). In this context we usually think about the temperature at which boiling occurs. For example, water boils at 100°C at sea-level atmospheric pressure (1 atm abs). However, in terms of vapor pressure, we can say that by increasing the temperature of water at sea level to 100 C, we increase the vapor pressure to the point at which it is equal to the atmospheric pressure (1 atm abs), so that boiling occurs. It is easy to visualize that boiling can also occur in water at temperatures much below 100°C if the pressure in the water is reduced to its vapor pressure. For example, the vapor pressure of water at 10°C is 0. 01 atm. Therefore, if the pressure (absolute pressure) within water at that temperature is reduced to that value, the water boils. Such boiling often occurs in flowing liquids, such as on the suction side of a pump or tip regions of impellers. When such boiling does occur in the flowing liquids, vapor bubbles start growing in local regions of very low pressure (high velocity) and then collapse in regions of high downstream pressure (low velocity). This phenomenon is called as cavitation.

Cavitation is not desirable for several reasons. First, it causes noise (as the cavitation bubbles collapse when they migrate into regions of higher pressure). Second, it can lead to inefficiencies and reduction of heat transfer in pumps and turbines (turbomachines). Finally, the collapse of these cavitation bubbles causes pitting and corrosion of blades and other surfaces nearby.

cavitation damage on a blade cavitating propeller in a water tunnel

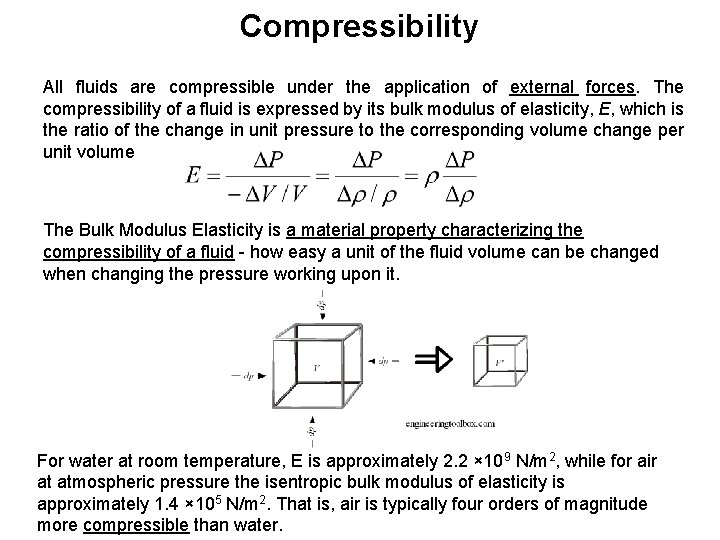
Compressibility All fluids are compressible under the application of external forces. The compressibility of a fluid is expressed by its bulk modulus of elasticity, E, which is the ratio of the change in unit pressure to the corresponding volume change per unit volume The Bulk Modulus Elasticity is a material property characterizing the compressibility of a fluid - how easy a unit of the fluid volume can be changed when changing the pressure working upon it. For water at room temperature, E is approximately 2. 2 × 109 N/m 2, while for air at atmospheric pressure the isentropic bulk modulus of elasticity is approximately 1. 4 × 105 N/m 2. That is, air is typically four orders of magnitude more compressible than water.

For most practical purposes liquids may be regarded as incompressible. However, there are certain cases, such as unsteady flow in pipes (e. g. , water hammer), where the compressibility should betaken into account. Gases may also be treated as incompressible if the change in density is very small (typically less than 3%). An ideal fluid is an incompressible fluid water hammer moving through pipes Expansion joints on a steam line that have been destroyed by steam hammer