Camp test Rhodococcus equi Staph aureus L Monocytogenes







































- Slides: 42







Camp test Rhodococcus equi Staph. aureus L. Monocytogenes - + L. Ivanovii + -



encephalitis אנצפליטיס




zipper mechanism • zippering of the host cell membrane around the bacterium as it enters. • This mode of entry into non-professional phagocytes is employed by Yersinia pseudotuberculosis and Listeria monocytogenes. • Bacterial ligand interacts with a surface molecule on the host cell. • The receptor is generally a protein involved in cell adhesion and/or activation of the cytoskeleton machinery. • The ligand-receptor interaction induces local rearrangements in actin cytoskeleton and other signals that culminate in the tight envelopment of the bacterial body by the plasma membrane.

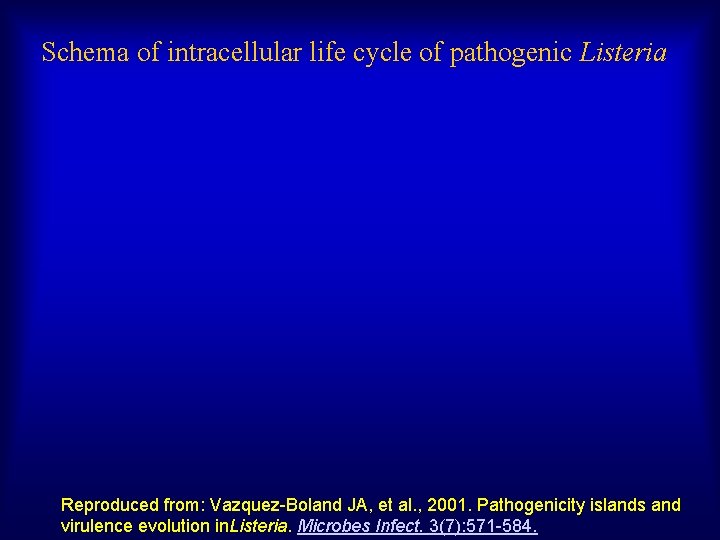
Schema of intracellular life cycle of pathogenic Listeria Reproduced from: Vazquez-Boland JA, et al. , 2001. Pathogenicity islands and virulence evolution in. Listeria. Microbes Infect. 3(7): 571 -584.

Virulence Factors • Actin-based motility Act. A • Adherence Ami Fbp. A • Bile resistance BSH • Exoenzyme Mpl Plc. A Plc. B SMase (L. ivanovii) • Intracellular growth Hpt Lpl. A 1 Svp. A • Invasion Auto Inl. A Inl. B Lpe. A p 60 • Peptidase Lsp • Regulation Prf. A • Stress protein Clp. C Clp. E Clp. P • Toxin LLO LLS

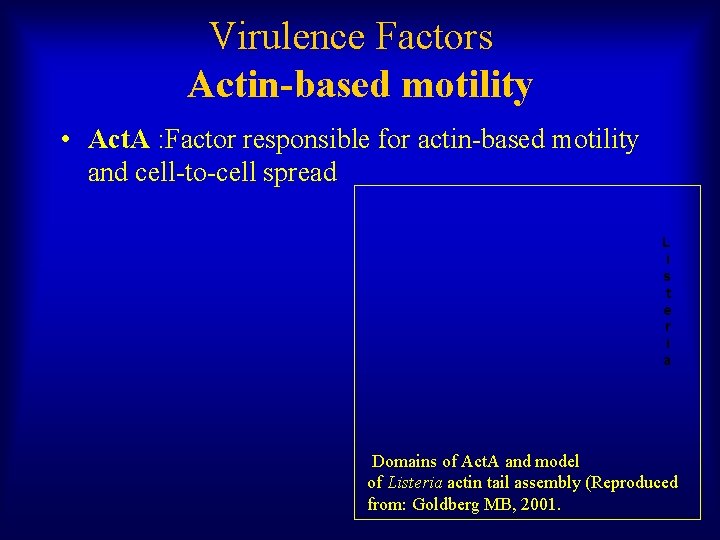
Virulence Factors Actin-based motility • Act. A : Factor responsible for actin-based motility and cell-to-cell spread L i s t e r i a Domains of Act. A and model of Listeria actin tail assembly (Reproduced from: Goldberg MB, 2001.

Virulence Factors • Actin-based motility Act. A • Adherence Ami Fbp. A • Bile resistance BSH • Exoenzyme Mpl Plc. A Plc. B SMase (L. ivanovii) • Intracellular growth Hpt Lpl. A 1 Svp. A • Invasion Auto Inl. A Inl. B Lpe. A p 60 • Peptidase Lsp • Regulation Prf. A • Stress protein Clp. C Clp. E Clp. P • Toxin LLO LLS

Virulence Factors Adherence • Ami : plays a role in the adhesion of L. monocytogenes to eukaryotic cells via its cell wallbinding domain. • Fbp. A : A fibronectin-binding protein present on the listerial surface that can mediate adherence to host cells but also act like a chaperone for two virulence factors, LLO and Inl. B

Virulence Factors • Actin-based motility Act. A • Adherence Ami Fbp. A • Bile resistance BSH • Exoenzyme Mpl Plc. A Plc. B SMase (L. ivanovii) • Intracellular growth Hpt Lpl. A 1 Svp. A • Invasion Auto Inl. A Inl. B Lpe. A p 60 • Peptidase Lsp • Regulation Prf. A • Stress protein Clp. C Clp. E Clp. P • Toxin LLO LLS

Virulence Factors • Actin-based motility Act. A • Adherence Ami Fbp. A • Bile resistance BSH • Exoenzyme Mpl Plc. A Plc. B SMase (L. ivanovii) • Intracellular growth Hpt Lpl. A 1 Svp. A • Invasion Auto Inl. A Inl. B Lpe. A p 60 • Peptidase Lsp • Regulation Prf. A • Stress protein Clp. C Clp. E Clp. P • Toxin LLO LLS

Virulence Factors Intracellular growth • Hpt (Hexose phosphate transporter) The mammalian G 6 PT is responsible for the uptake of G 6 P from the cytosol into the endoplasmic reticulum for its conversion into glucose. • Hpt mimics the function of the mammalian G 6 PT to steal fueling metabolites from host cell cytosol for the benefit of the microbe. • vp. A (surface-virulence associated protein) A novel factor may be involved in promoting bacterial escape from phagosomes of bacteria

Virulence Factors • Actin-based motility Act. A • Adherence Ami Fbp. A • Bile resistance BSH • Exoenzyme Mpl Plc. A Plc. B SMase (L. ivanovii) • Intracellular growth Hpt Lpl. A 1 Svp. A • Invasion Auto Inl. A Inl. B Lpe. A p 60 • Peptidase Lsp • Regulation Prf. A • Stress protein Clp. C Clp. E Clp. P • Toxin LLO LLS

Virulence Factors Invasion • Auto : surface protein in L. monocytogenes but absent in L. innocua Required for entry of L. monocytogenes into nonphagocytic cells and necessary for full virulence • Inl. A (Internalin), Inl. B : Promote entry into host cells. Mediate the crossing of the intestinal and placental barriers, and invasion of the central nervous system (CNS) may also be mediated by the interaction between Inl. A and E-cadherin.

Virulence Factors • Actin-based motility Act. A • Adherence Ami Fbp. A • Bile resistance BSH • Exoenzyme Mpl Plc. A Plc. B SMase (L. ivanovii) • Intracellular growth Hpt Lpl. A 1 Svp. A • Invasion Auto Inl. A Inl. B Lpe. A p 60 • Peptidase Lsp • Regulation Prf. A • Stress protein Clp. C Clp. E Clp. P • Toxin LLO LLS

Virulence Factors Regulation Prf. A (Pleiotropic regulatory factor) • coordinates regulation of the virulence factors and is temperature controlled • Influenced by environment: temp. , p. H, nutrients etc. • The main switch of a regulon including the majority of the known listerial virulence genes: LIPI-1 genes (with prf. A itself) and several genes of the subfamily of secreted internalins (e. g. , inl. C of L. monocytogenes and i-inl. E of L. ivanovii) • Prf. A negatively regulates stress response mediator gene clp. C and the motility-associated genes mot. A and fla. A

Virulence Factors • Actin-based motility Act. A • Adherence Ami Fbp. A • Bile resistance BSH • Exoenzyme Mpl Plc. A Plc. B SMase (L. ivanovii) • Intracellular growth Hpt Lpl. A 1 Svp. A • Invasion Auto Inl. A Inl. B Lpe. A p 60 • Peptidase Lsp • Regulation Prf. A • Stress protein Clp. C Clp. E Clp. P • Toxin LLO LLS

Virulence Factors Stress protein • Clp. C : An ATPase promoting early escape form the phagosome of macrophages – also required for adhesion and invasion, possibly by modulating the expression of Inl. A, Inl. B and Act. A • Clp. E : An ATPase required for prolonged survival at 42 degree Acts synergistically with Clp. C in cell division • Clp. P : Serine protease involved in proteolysis and is required for growth under stress conditions

Virulence Factors • Actin-based motility Act. A • Adherence Ami Fbp. A • Bile resistance BSH • Exoenzyme Mpl Plc. A Plc. B SMase (L. ivanovii) • Intracellular growth Hpt Lpl. A 1 Svp. A • Invasion Auto Inl. A Inl. B Lpe. A p 60 • Peptidase Lsp • Regulation Prf. A • Stress protein Clp. C Clp. E Clp. P • Toxin LLO LLS

Virulence Factors Toxin • LLO (Listeriolysin O) : Mediates lysis of the primary phagosomes formed after the uptake of extracellular bacteria, and required for the efficient escape from the double-membrane vacuole that forms upon cell-to-cell spead The pores or membrane lesions caused by LLO facilitate the access of Listeria phospholipases to their substrates, leading to total dissolution of the physical barrier that delimits the phagosomal compartment Multifunctional virulence factor: induce a number of host cell responses, such as cell proliferation, activation of MAP kinase pathway in epithelial cells, modulation of internalization via calcium signaling and cytokine expression in macrophages, etc.












Listeria monocytogenes infection in Israel and review of cases worldwide Emerging Infectious Diseases , March, 2002 by Yardena Siegman-Igra , Rotem Levin , Miriam Weinberger , Yoav Golan , David Schwartz , Zmira Samra , Hana Konigsberger , Amos Yinnon , Galia Rahav , Nathan Keller , Nail Bisharat , Jehuda Karpuch , Renato Finkelstein , Michael Alkan , Zvi Landau , Julia Novikov , David Hassin , Carlos Rudnicki , Ruth Kitzes , Shmouel Ovadia , Zvi Shimoni , Ruth Lang , Tamar Shohat Listeria monocytogenes, an uncommon foodborne pathogen, is increasingly recognized as a cause of life-threatening disease. A marked increase in reported cases of listeriosis during 1998 motivated a retrospective nationwide survey of the infection in Israel. From 1995 to 1999, 161 cases were identified; 70 (43%) were perinatal infections, with a fetal mortality rate of 45%. Most (74%) of the 91 nonperinatal infections involved immunocompromised patients with malignancies, chronic liver disease, chronic renal failure, or diabetes mellitus. The common clinical syndromes in these patients were primary bacteremia (47%) and meningitis (28%). The crude case-fatality rate in this group was 38%, with a higher death rate in immunocompromised patients.