Microscope Microscope The Simple Stain l In a
























- Slides: 41

Microscope

Microscope

The Simple Stain l In a simple stain, the smear is stained with a solution of a single dye which stains all cells the same color. Differentiation of cell types or structures is not the objective of the simple stain. However, certain structures which are not stained by this method may be easily seen, for example, endospores and lipid inclusions.

Procedure l Prepare and heat-fix a smear of the organism to be studied. l Cover the smear with the staining solution. If crystal violet or safranin is used, allow one minute for staining. The use of methylene blue requires 3 -5 minutes to achieve good staining. l Carefully wash off the dye with tap water and blot the slide dry with blotting paper, an absorbent paper pad or a paper towel.

Figure : The Simple Stain

Gram Stain l The Gram stain, performed properly, differentiates nearly all bacteria into two major groups. For example, one group, the grampositive bacteria, include the causative agents of the diseases diphtheria, anthrax, tetanus, scarlet fever, and certain forms of pneumonia and tonsillitis. A second group, the gram-negative bacteria, includes organisms which cause typhoid fever, dysentery, gonorrhea and whooping cough. In Bacteria the reaction to Gram stain reagents is explained by different cell wall structures. Grampositive microbes have a much thicker cell wall, while that found in Gram-negative microbes is thinner. Microbes from the Archaea domain contain different cell wall structures than that seen in microbes commonly found in the lab (Bacteria domain). However, they will still have a species specific Gram stain reaction, even though the underlying macromolecular structures are different.

The Gram stain is one of the most useful differential stains in bacteriology, including diagnostic medical bacteriology. The differential staining effect correlates to differences in the cell wall structure of microorganisms (at least Bacteria, but not Archaea as mentioned above). In order to obtain reliable results it is important to take the following precautions: l The cultures to be stained should be young - incubated in broth or on a solid medium until growth is just visible (no more than 12 to 18 hours old if possible). Old cultures of some gram-positive bacteria will appear Gram negative. This is especially true for endospore-forming bacteria, such as species from the genus Bacillus. In this class, many of the cultures will have grown for more than 2 days. For most bacteria this is not a problem, but be aware that some cultures staining characteristics may change! l When feasible, the cultures to be stained should be grown on a sugarfree medium. Many organisms produce substantial amounts of capsular or slime material in the presence of certain carbohydrates. This may interfere with decolorization, and certain Gram-negative organisms such as Klebsiella may appear as a mixture of pink and purple cells. l

Gram stain procedure l l l Below is a procedure that works well in the teaching laboratories. Cover the slide with crystal violet stain and wait one minute. After one minute wash the stain off (gently!) with a minimum amount of tap water. Drain off most of the water and proceed to the next step. It may help to hold the slide vertically and touch a bottom corner to paper toweling or blotting paper. Cover the slide with iodine solution for one minute. The iodine acts as a mordant (fixer) and will form a complex with the crystal violet, fixing it into the cell. Rinse briefly with tap water.

Tilt the slide lengthwise over the sink and apply the alcoholacetone decolorizing solution (dropwise) such that the solution washes over the entire slide from one end to the other. All smears on the slide are to be treated thoroughly and equally in this procedure. Process the sample in this manner for about 25 seconds and immediately rinse with tap water. This procedure will decolorize cells with a Gram negative type of cell wall but not those with a gram-positive type of cell wall, as a general rule. Drain off most of the water and proceed. l As the decolorized gram-negative cells need to be stained in order to be visible, cover the slide with the safranin counterstain for 30 seconds to one minute. l Rinse briefly and blot the slide dry. Record each culture as Gram positive (purple cells) or Gram negative (pink cells). l

Gram Stain Procedure

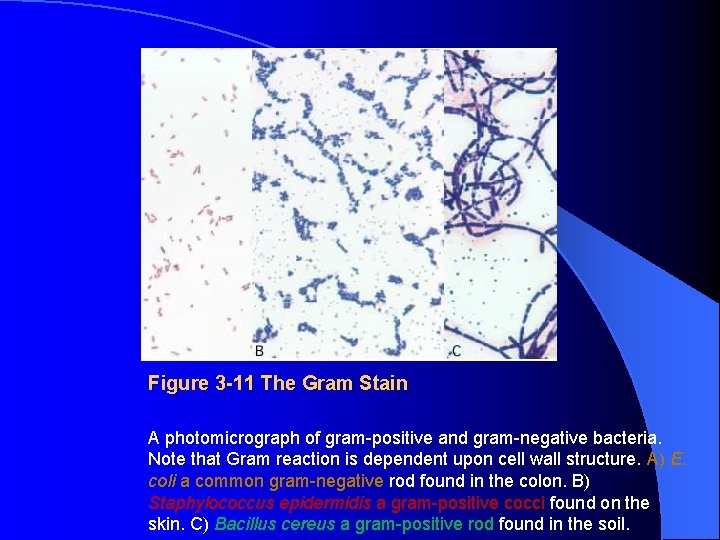
Figure 3 -11 The Gram Stain A photomicrograph of gram-positive and gram-negative bacteria. Note that Gram reaction is dependent upon cell wall structure. A) E. coli a common gram-negative rod found in the colon. B) Staphylococcus epidermidis a gram-positive cocci found on the skin. C) Bacillus cereus a gram-positive rod found in the soil.

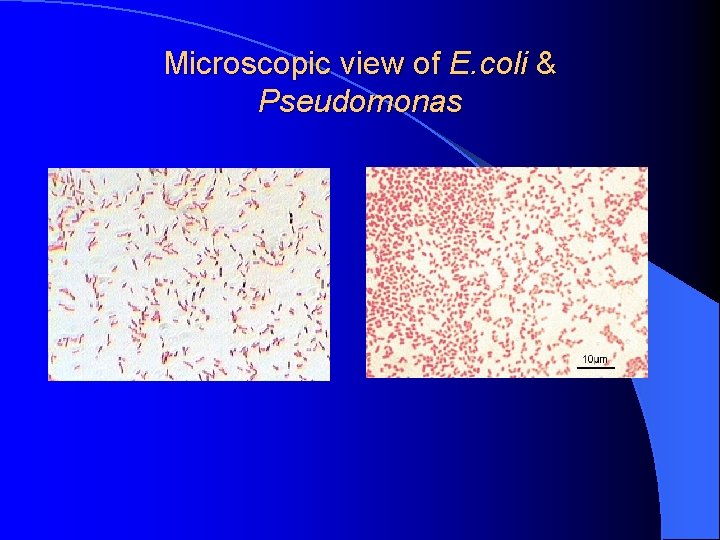
Microscopic view of E. coli & Pseudomonas

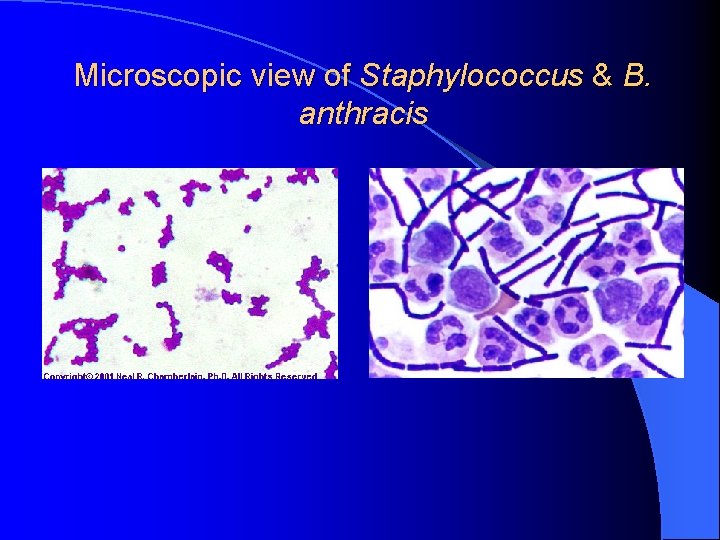
Microscopic view of Staphylococcus & B. anthracis

The Endospore Stain Cells of Bacillus, Desulfotomaculum and Clostridium (and several other, lesser-known genera--see Bergey's Manual) may, as a response to nutrient limitations, develop endospores that possess remarkable resistance to heat, dryness, irradiation and many chemical agents. Each cell can produce only one endospore. It is therefore not a reproductive spore as seen for some organisms such as Streptomyces and most molds. The endospore is essentially a specialized cell, containing a full complement of DNA and many proteins, but little water. This dehydration contributes to the spores resistance and makes it metabolically inert. The endospore develops in a characteristic position (for its species) in the vegetative cell. Eventually the cell lyses, releasing a free endospore.

Endospore Stain Procedure l Endospore stains require heat to drive the stain into the cells. For a endospore stain to be successful, the temperature of the stain must be near boiling and the stain cannot dry out. Most failed endospore stains occur because the stain was allowed to completely evaporate during the procedure.

Place the heat-fixed slide over a steaming water bath and place a piece of blotting paper over the area of the smear. The blotting paper should completely cover the smear, but should not stick out past the edges of the slide. If it sticks out over the edges stain will flow over the edge of the slide by capillary action and make a mess. l Saturate the blotting paper with the 5 -6% solution of malachite green. Allow the steam to heat the slide for five minutes, and replenish the stain if it appears to be drying out. l Cool the slide to room temperature. Rinse thoroughly and carefully with tap water. l

l Apply safranin for one minute. Rinse thoroughly but briefly with tap water, blot dry and examine. Mature endospores stain green whether free or in the vegetative cell. Vegetative cells stain pink to red.

Figure: The Endospore Stain A photomicrograph of an enodspore stain. Spores present in the picture stain green, while the vegetative cells stain red. A) Staphylococcus epdiermidis which does not form endospores. B) The endospore-forming rod, Bacillus cereus.

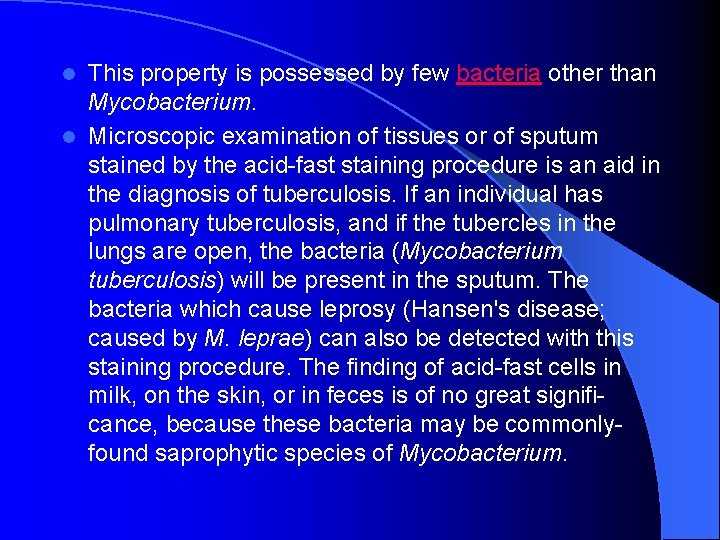
The Acid fast Stain l Because of the waxy substance (mycolic acids) present on the cell walls, cells of species of Mycobacterium do not stain readily with ordinary dyes. However, treatment with cold carbol fuchsin for several hours or at high temperatures for five minutes will dye the cells. Once the cells have been stained, subsequent treatment with a dilute hydrochloric acid solution or ethyl alcohol containing 3% HCl (acidalcohol) will not decolorize them. Such cells are thus termed acid-fast in that the cell will hold the stain fast in the presence of the acidic decolorizing agent. This property is possessed by few bacteria other than Mycobacterium.

This property is possessed by few bacteria other than Mycobacterium. l Microscopic examination of tissues or of sputum stained by the acid-fast staining procedure is an aid in the diagnosis of tuberculosis. If an individual has pulmonary tuberculosis, and if the tubercles in the lungs are open, the bacteria (Mycobacterium tuberculosis) will be present in the sputum. The bacteria which cause leprosy (Hansen's disease; caused by M. leprae) can also be detected with this staining procedure. The finding of acid-fast cells in milk, on the skin, or in feces is of no great significance, because these bacteria may be commonlyfound saprophytic species of Mycobacterium. l

After preparation of the heat-fixed smear, place the slide over a steaming water bath. l Place a piece of paper towel or blotting paper over the smear. The paper should be about as wide as the slide and cover an area just slightly greater than the smear itself. Saturate the paper with carbol fuchsin and let the slide remain above the steaming water bath for five minutes. Add more carbol fuchsin to the paper if it appears the stain is drying out. l Allow the slide to cool to room temperature. Remove the paper and wash off the excess stain with water. l

Decolorize the smear with acid-alcohol for 1015 seconds. Wash gently with tap water. l Counterstain with methylene blue for 3 minutes. Rinse the slide gently and dry. l Examine the smear first with the 10 X and then the 100 X (oil-immersion) objective. Those cells which retained the primary stain (carbol fuchsin) through the acid-alcohol treatment are stained red; these are the acid-fast organisms. Mycobacterium cells characteristically appear as clusters of long, red rods. All other cells are blue. l

Figure: The acid fast stain A photomicrograph of Mycobacterium smegmatis (pink) and Micrococcus luteus (blue) at 1000 x magnification. M. smegmatis is acid-fast, retaining the carbol fuchsin dye, thus appearing pink. M. luteus is not acid-fast, loses the carbol fuchsin during decolorizaiton, and is counter-stained with methylene blue.

Microscopic Observation of Stained Cell Preparation

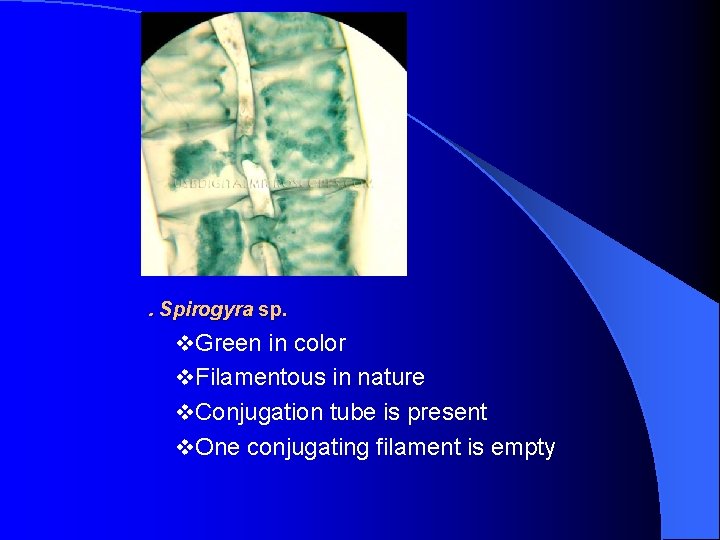
. Spirogyra sp. v. Green in color v. Filamentous in nature v. Conjugation tube is present v. One conjugating filament is empty

Volvox sp. ØSpherical colony of green alga Volvox ØSingle celled flagellates embedded in a gelatinous matrix and organized into a hollow sphere. ØThe indivisual cells are joined by cytoplasmic threads. ØEach parental colony has a number of developing projeny colonies, which are formed by repeated divison of a few specialized reproductive cells ØProjeny colonies are released through disintegration of the parental colony.

Penicillium sp. v. The mycelium is septed, long and branched v. The conidiophores branched about twothirds of the way to the tip in broom-like fashion v. Single celled conidia developed at the end of sterigma in chains v. The conidia are globose to ovoid and green in color

Aspergillus sp. a. The hyphae were well developed, profusely branched and septed. b. The conidiophore formed a bulbous head, the vesicle. c. Conidia arose from sterigma, at their tips in a chain. d. Conidia were typically globose, unicellular, enormous and black in color.

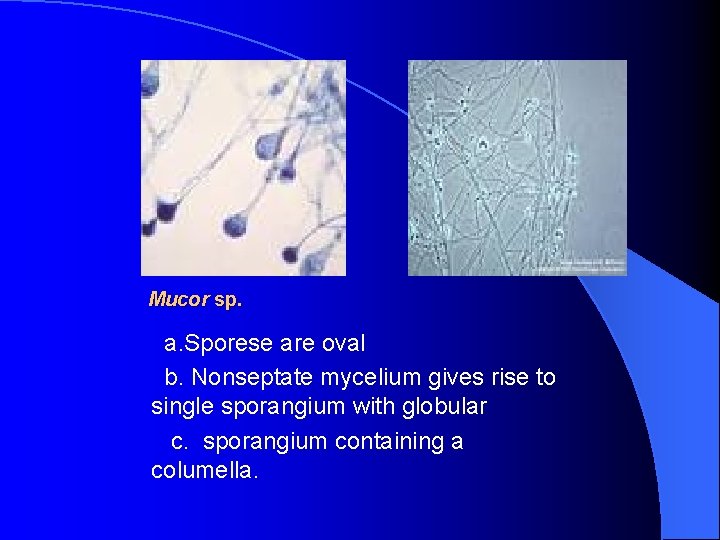
Mucor sp. a. Sporese are oval b. Nonseptate mycelium gives rise to single sporangium with globular c. sporangium containing a columella.

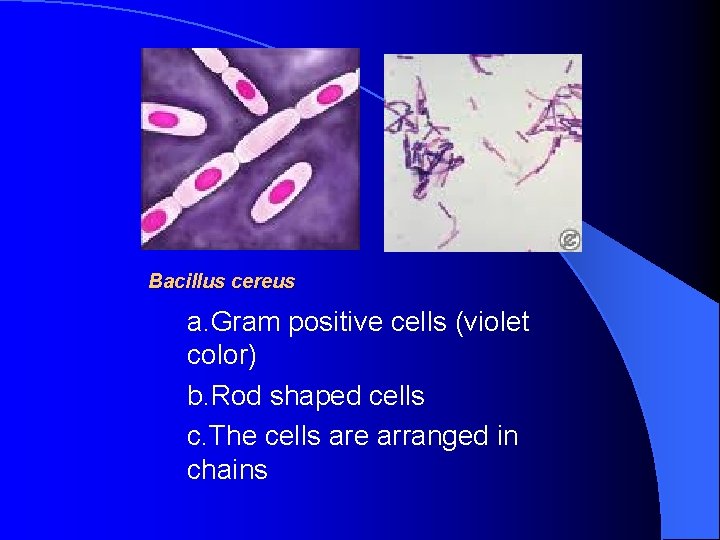
Bacillus cereus a. Gram positive cells (violet color) b. Rod shaped cells c. The cells are arranged in chains

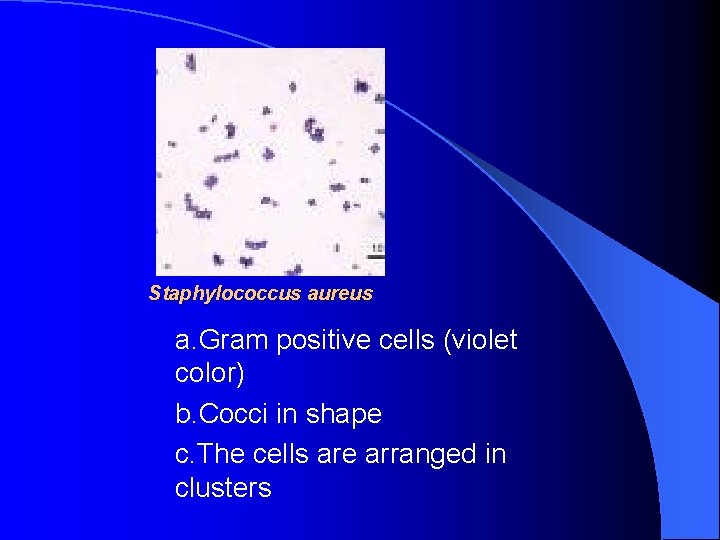
Staphylococcus aureus a. Gram positive cells (violet color) b. Cocci in shape c. The cells are arranged in clusters

Selective & Differential Media Selective Medium: culture medium that allows the growth of certain types of organisms, while inhibiting the growth of other organisms l dyes in the medium (e. g. : methylene blue in EMB & crystal violet in Mac. Conkey's) or high salt concentration in the medium (e. g. : 7% salt in MSA) inhibit the growth of unwanted microorganisms l Differential Medium: culture medium that allows one to distinguish between or among different microorganisms based on a difference in colony appearance (color, shape, or growth pattern) on the medium. l dyes in the medium (e. g. : eosin/methylene blue in EMB) or p. H indicators change the color of the medium as sugars in the medium (e. g. : lactose in EMB & Mac. Conkey's and mannitol in MSA) are fermented to produce acid products l

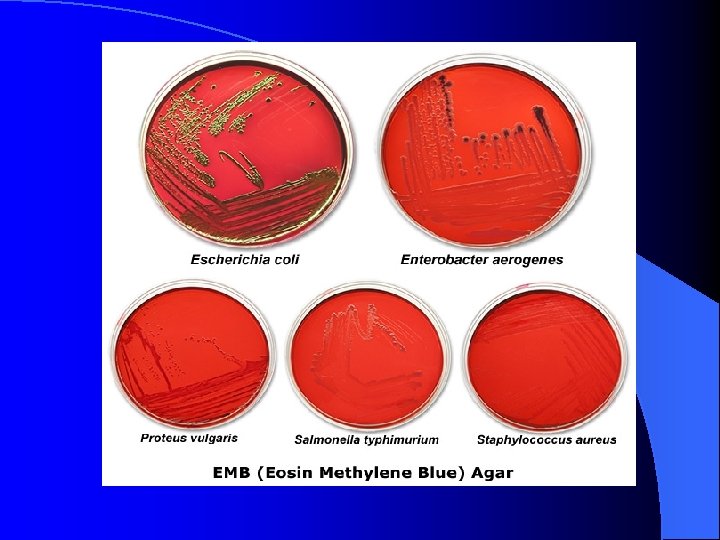
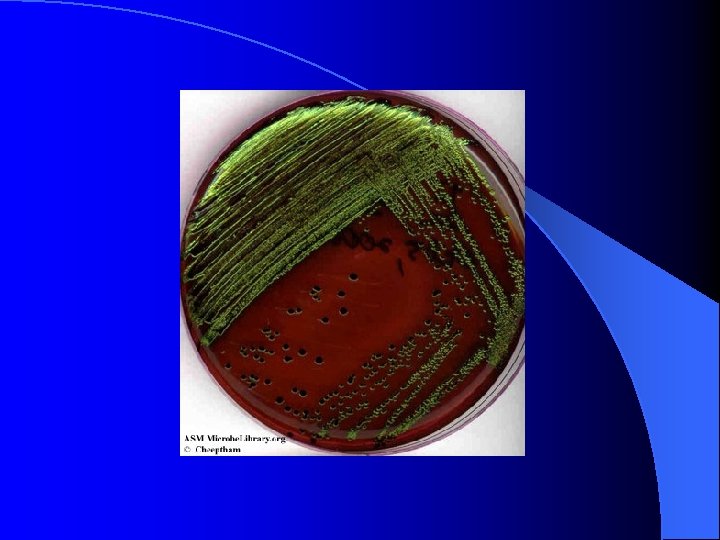
EMB (Eosin Methylene Blue) Agar l l l selective for: gram-negative bacteria growth of gram-positive bacteria (e. g. : Staphylococcus aureus in the image below) is inhibited by the eosin & methylene blue dyes in the media differential for: lactose fermentation gram-negative Enterobacteria Escherichia coli and Enterobacter aerogenes ferment lactose E. coli produces colonies with a characteristic green metallic sheen on EMB agar E. aerogenes produces pink colonies often with a central dark purple dot (fish eye colonies) on EMB agar gram-negative bacteria Proteus vulgaris and Salmonella typhimurium grow on EMB agar, but do not ferment lactose



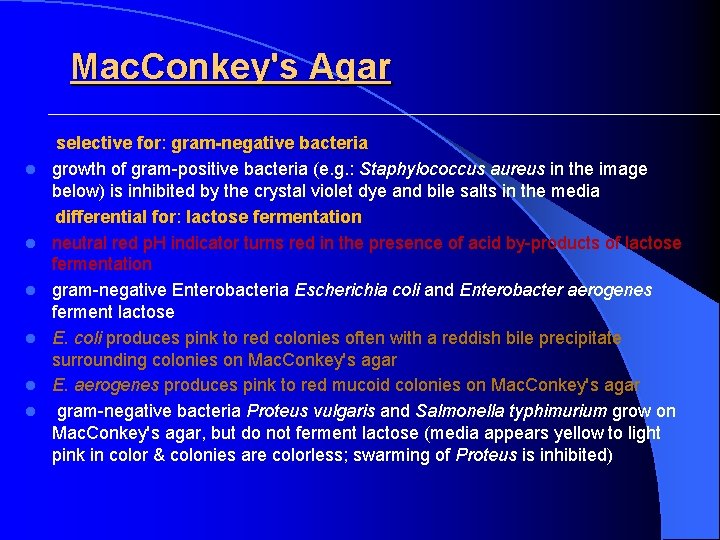
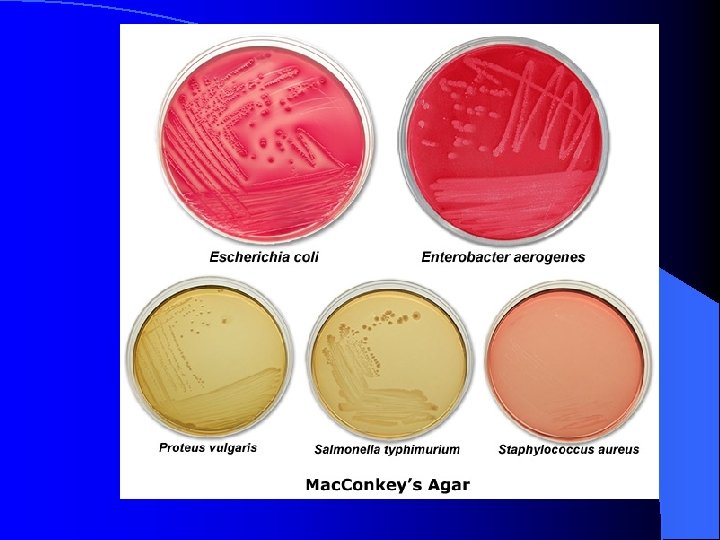
Mac. Conkey's Agar l l l selective for: gram-negative bacteria growth of gram-positive bacteria (e. g. : Staphylococcus aureus in the image below) is inhibited by the crystal violet dye and bile salts in the media differential for: lactose fermentation neutral red p. H indicator turns red in the presence of acid by-products of lactose fermentation gram-negative Enterobacteria Escherichia coli and Enterobacter aerogenes ferment lactose E. coli produces pink to red colonies often with a reddish bile precipitate surrounding colonies on Mac. Conkey's agar E. aerogenes produces pink to red mucoid colonies on Mac. Conkey's agar gram-negative bacteria Proteus vulgaris and Salmonella typhimurium grow on Mac. Conkey's agar, but do not ferment lactose (media appears yellow to light pink in color & colonies are colorless; swarming of Proteus is inhibited)


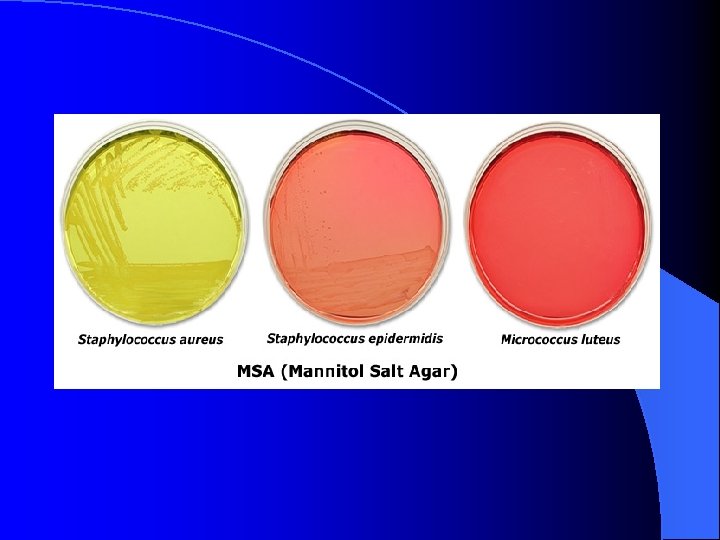
MSA (Mannitol Salt Agar) l l l selective for: gram-positive Staphylococci bacteria 7% salt in the medium inhibits the growth of most gram-positive and gram-negative bacteria differential for: mannitol fermentation phenol red p. H indicator turns yellow in the presence of acid byproducts of mannitol fermentation Staphylococcus aureus ferments mannitol S. aureus changes the color of the medium from pink to yellow due to acid by-products of mannitol fermentation Staphylococcus epidermidis grows on MSA, but does not ferment mannitol (media remains light pink in color & colonies are colorless


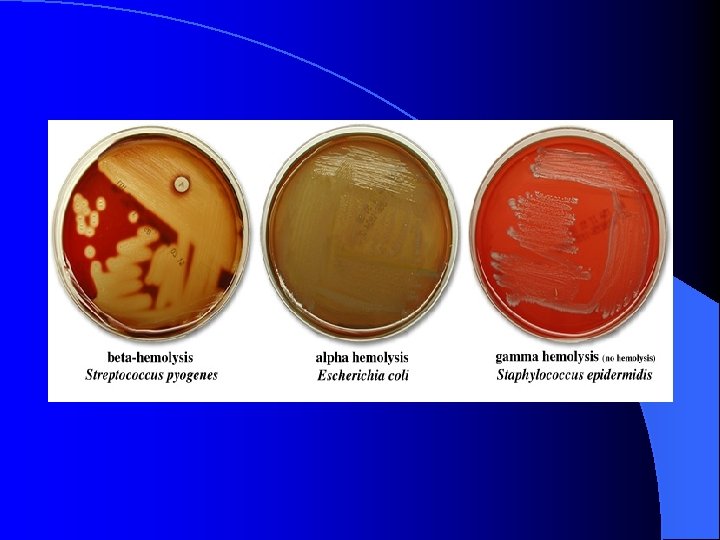
Hemolysis with Blood Agar l l l agar contains 5% sheep's blood differential for: hemolysis. . . particularly in streptococci based on the ability to break down hemoglobin or red blood cells, 3 groups of microorganisms can be described alpha-hemolysis: a green to light-brown halo is seen around the colonies; bacteria partially break down hemoglobin leaving a green pigment (biliverdin) beta-hemolysis: a clearing is seen around the colonies; bacteria produce a "beta-hemolysin" (streptolysin O or S), which lyses red blood cells in the medium gamma-hemolysis (no hemolysis): no hemolysis is observed; bacteria do not produce a hemolysin
