Atoms and Atomic Theory CHEMICAL SCIENCE Atoms Matter



- Slides: 18

Atoms and Atomic Theory CHEMICAL SCIENCE

Atoms • Matter is anything that takes up space and has mass • All matter is made of atoms • Atoms are the basic building blocks of matter • Atoms are too small to see without powerful microscopes.

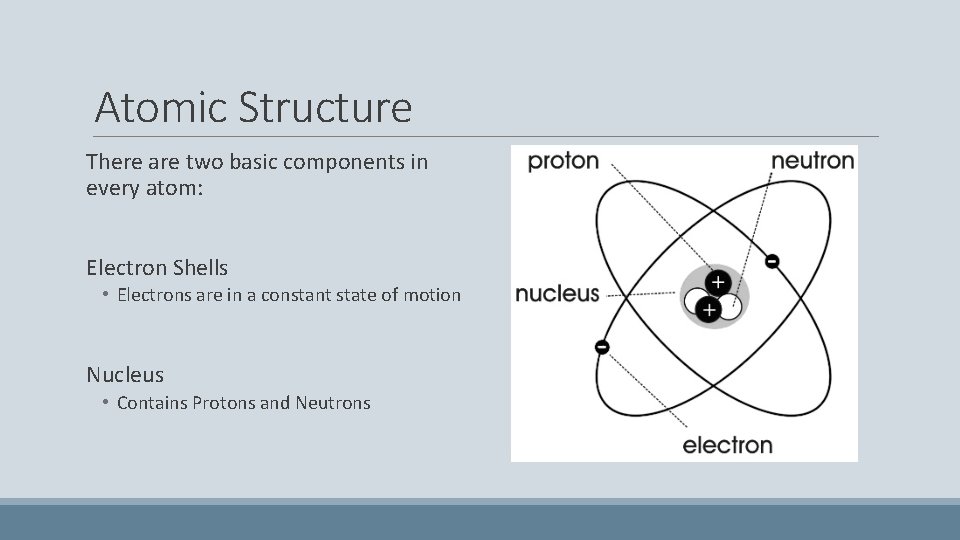
Atomic Structure There are two basic components in every atom: Electron Shells • Electrons are in a constant state of motion Nucleus • Contains Protons and Neutrons

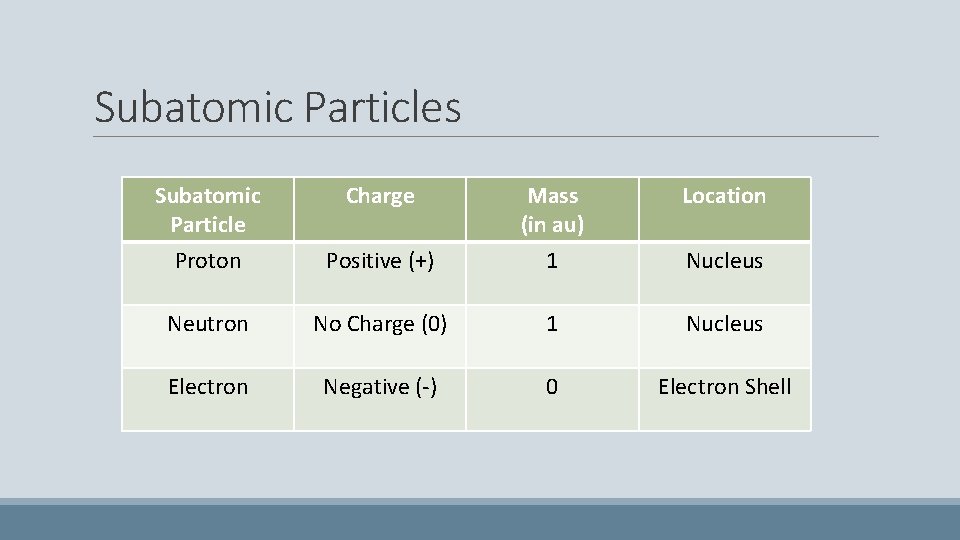
Subatomic Particles Subatomic Particle Charge Mass (in au) Location Proton Positive (+) 1 Nucleus Neutron No Charge (0) 1 Nucleus Electron Negative (-) 0 Electron Shell

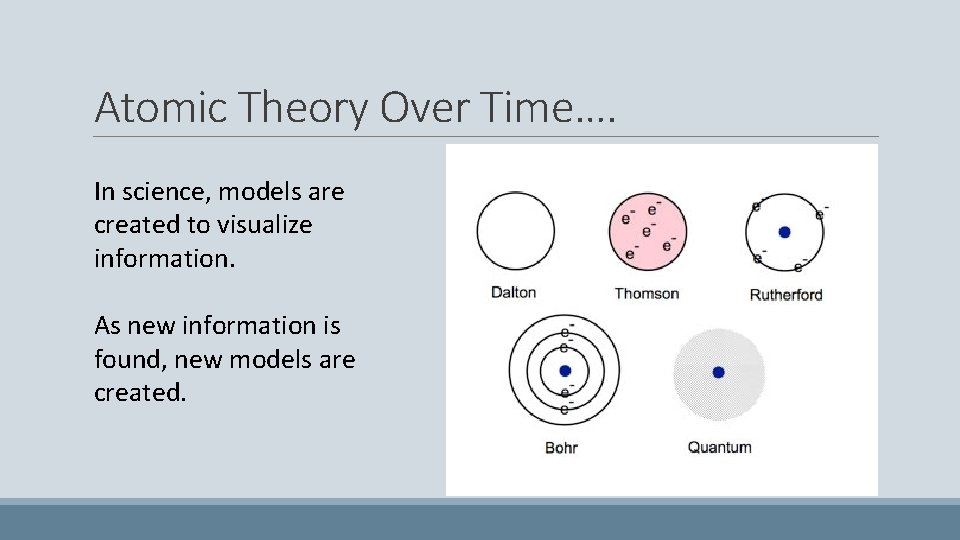
Atomic Theory Over Time…. In science, models are created to visualize information. As new information is found, new models are created.

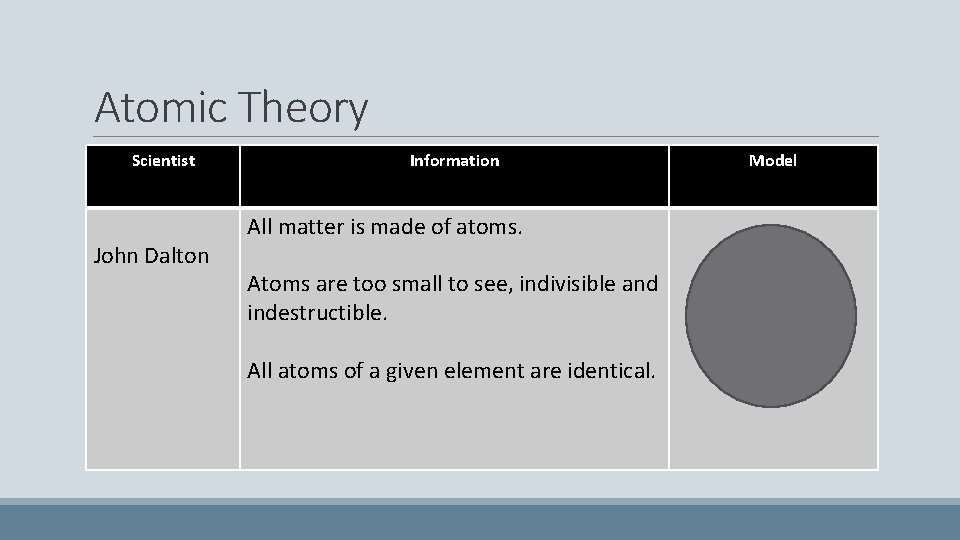
Atomic Theory Scientist John Dalton Information All matter is made of atoms. Atoms are too small to see, indivisible and indestructible. All atoms of a given element are identical. Model

Atomic Theory Scientist Information Discovered the negative electron, and predicted that there also must be a J. J Thompson positive particle to hold the electrons in place. Model

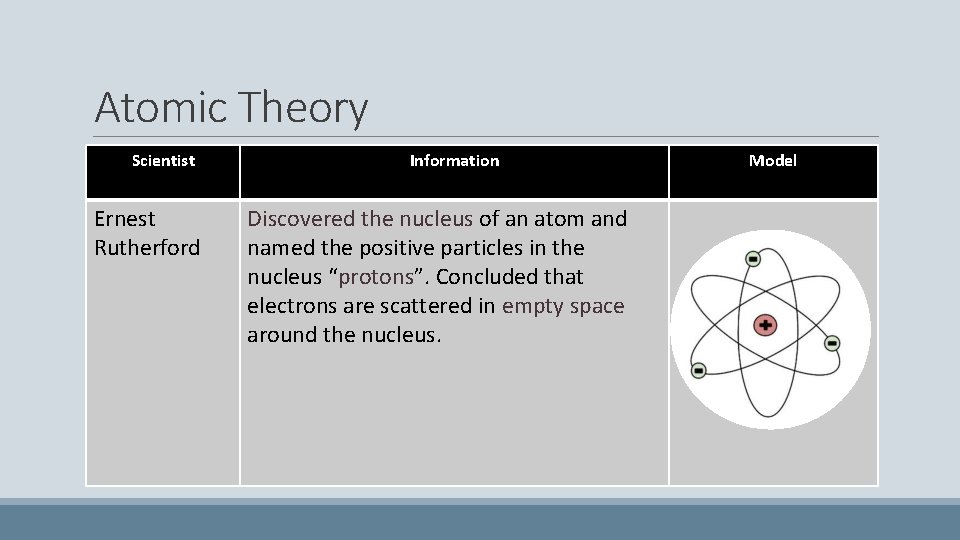
Atomic Theory Scientist Ernest Rutherford Information Discovered the nucleus of an atom and named the positive particles in the nucleus “protons”. Concluded that electrons are scattered in empty space around the nucleus. Model

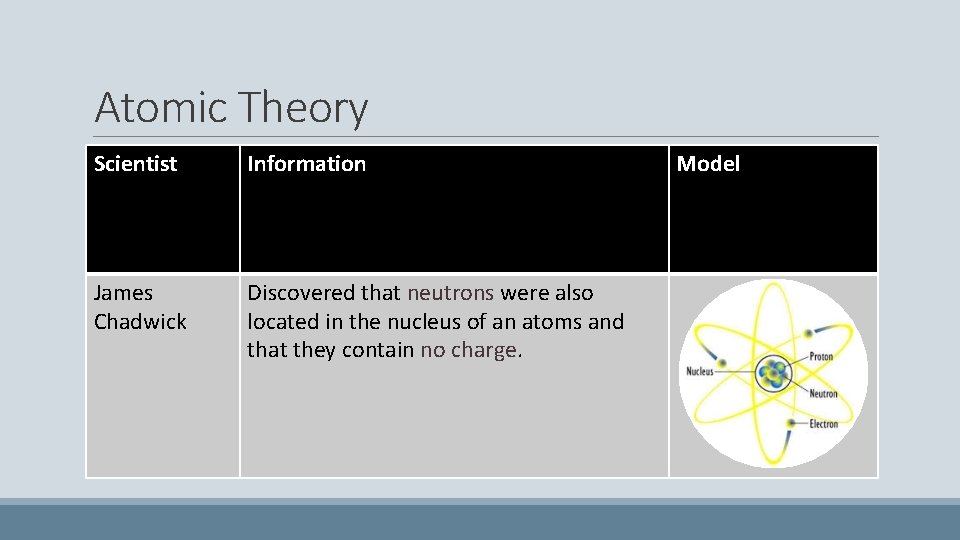
Atomic Theory Scientist Information James Chadwick Discovered that neutrons were also located in the nucleus of an atoms and that they contain no charge. Model

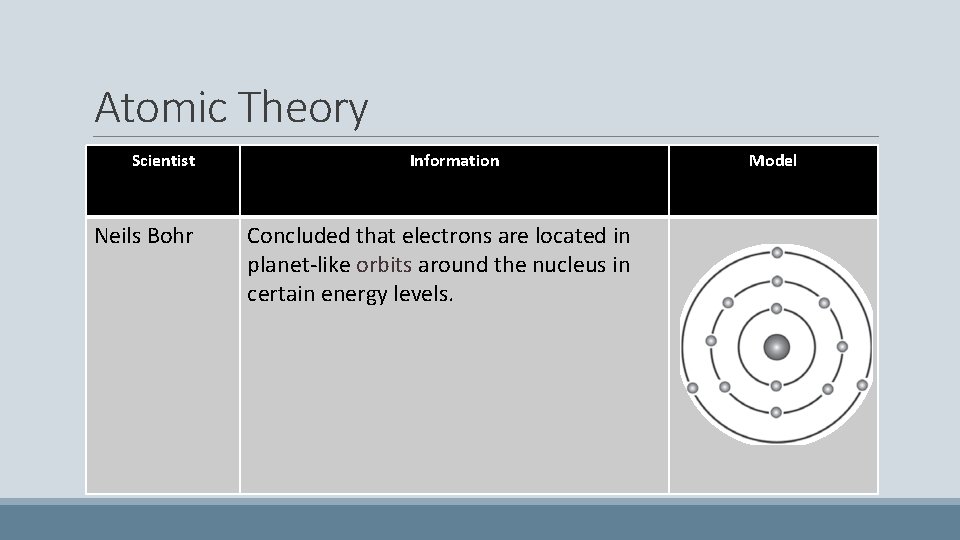
Atomic Theory Scientist Neils Bohr Information Concluded that electrons are located in planet-like orbits around the nucleus in certain energy levels. Model

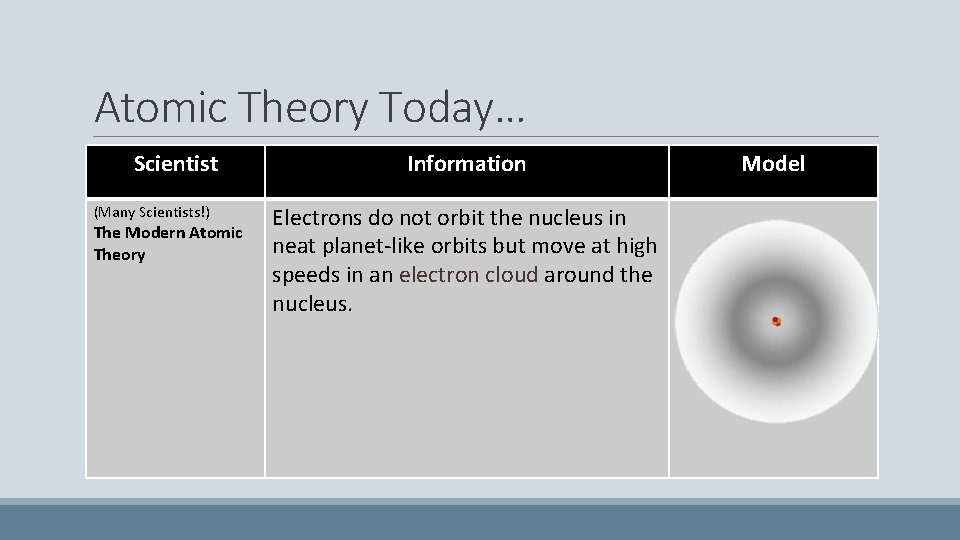
Atomic Theory Today… Scientist (Many Scientists!) The Modern Atomic Theory Information Electrons do not orbit the nucleus in neat planet-like orbits but move at high speeds in an electron cloud around the nucleus. Model

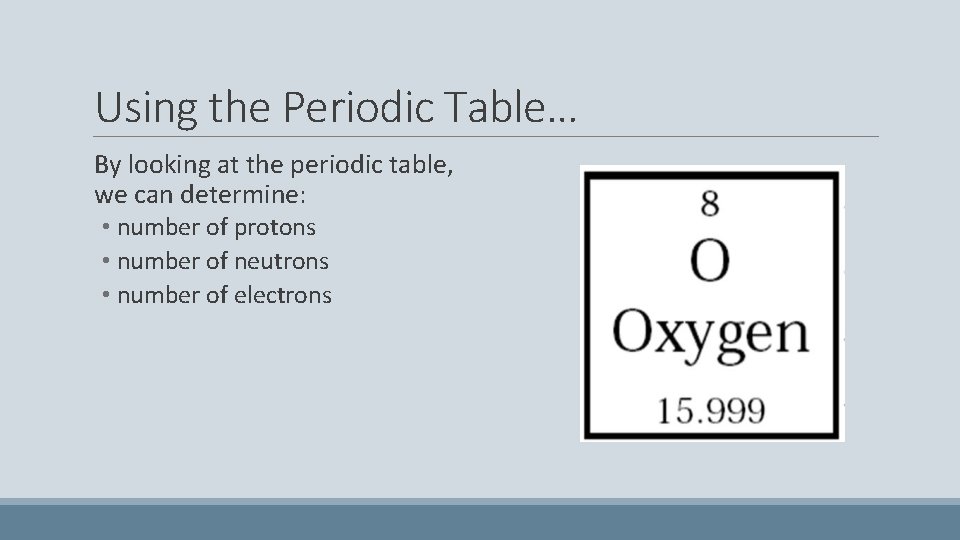
Using the Periodic Table… By looking at the periodic table, we can determine: • number of protons • number of neutrons • number of electrons

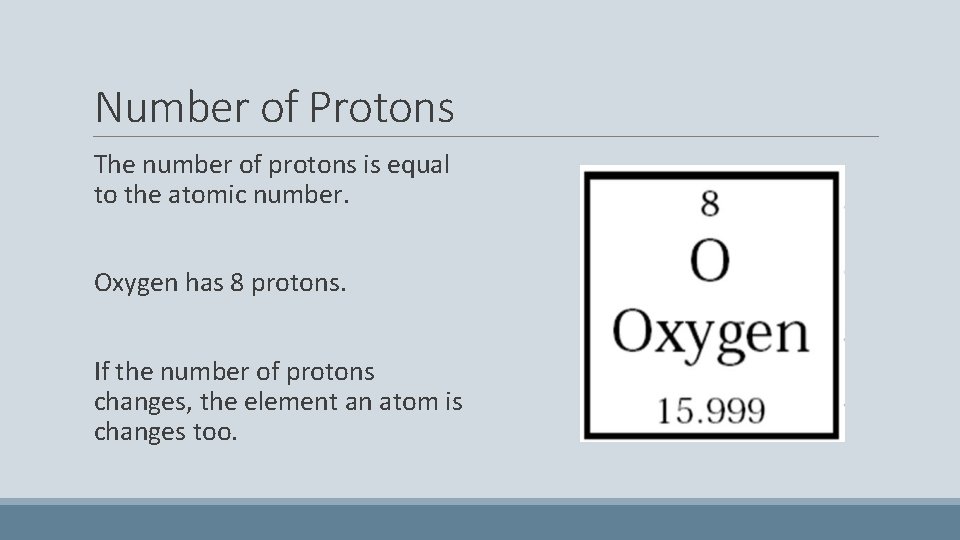
Number of Protons The number of protons is equal to the atomic number. Oxygen has 8 protons. If the number of protons changes, the element an atom is changes too.

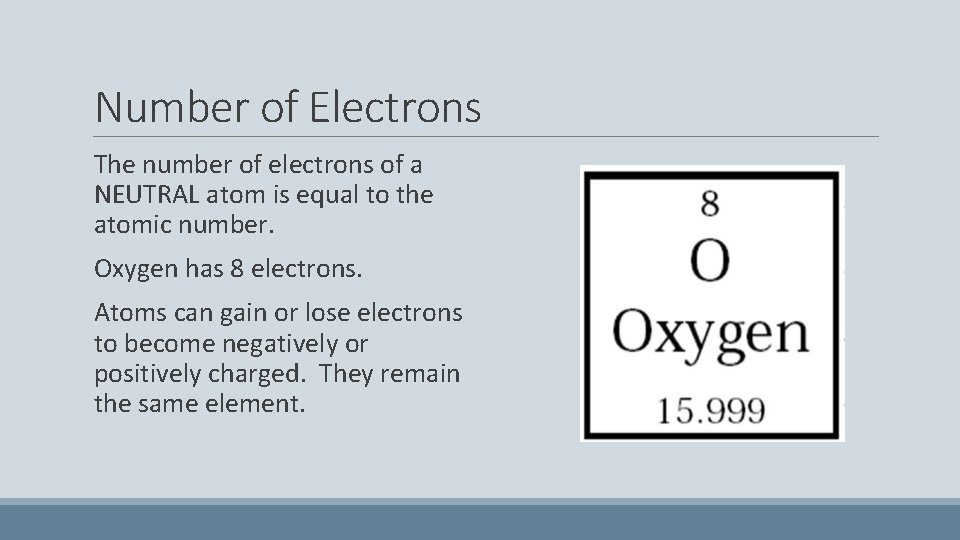
Number of Electrons The number of electrons of a NEUTRAL atom is equal to the atomic number. Oxygen has 8 electrons. Atoms can gain or lose electrons to become negatively or positively charged. They remain the same element.

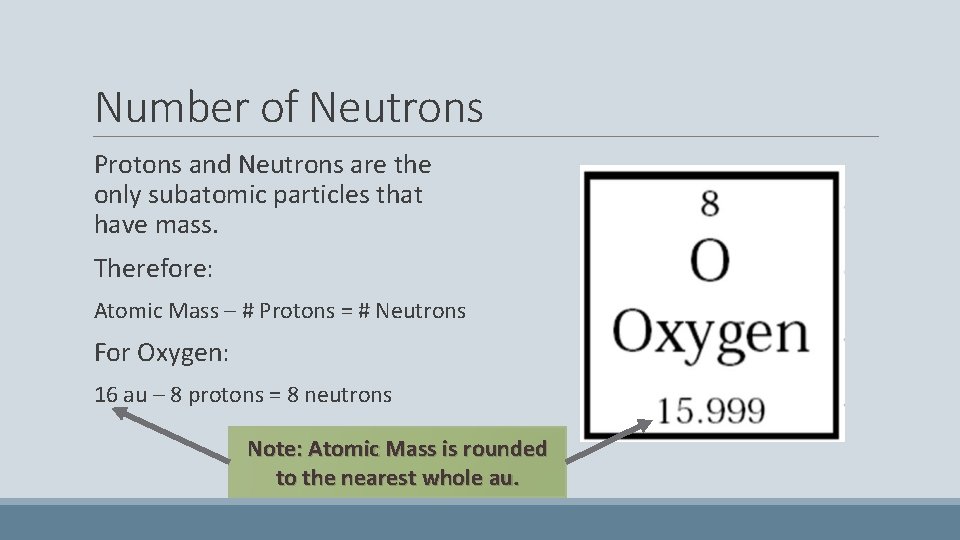
Number of Neutrons Protons and Neutrons are the only subatomic particles that have mass. Therefore: Atomic Mass – # Protons = # Neutrons For Oxygen: 16 au – 8 protons = 8 neutrons Note: Atomic Mass is rounded to the nearest whole au.

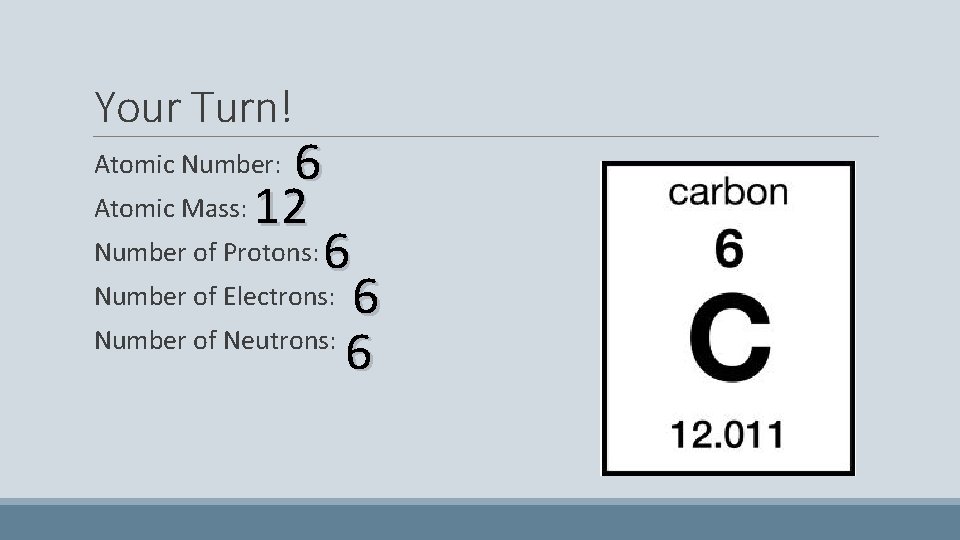
Your Turn! 6 Atomic Mass: 12 Number of Protons: 6 Number of Electrons: 6 Number of Neutrons: 6 Atomic Number:

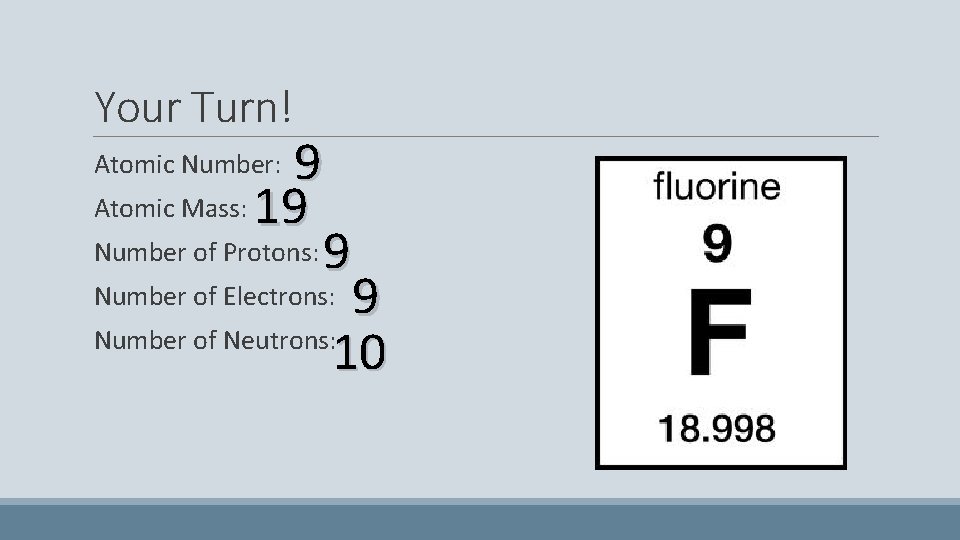
Your Turn! 9 Atomic Mass: 19 Number of Protons: 9 Number of Electrons: 9 Number of Neutrons: 10 Atomic Number:

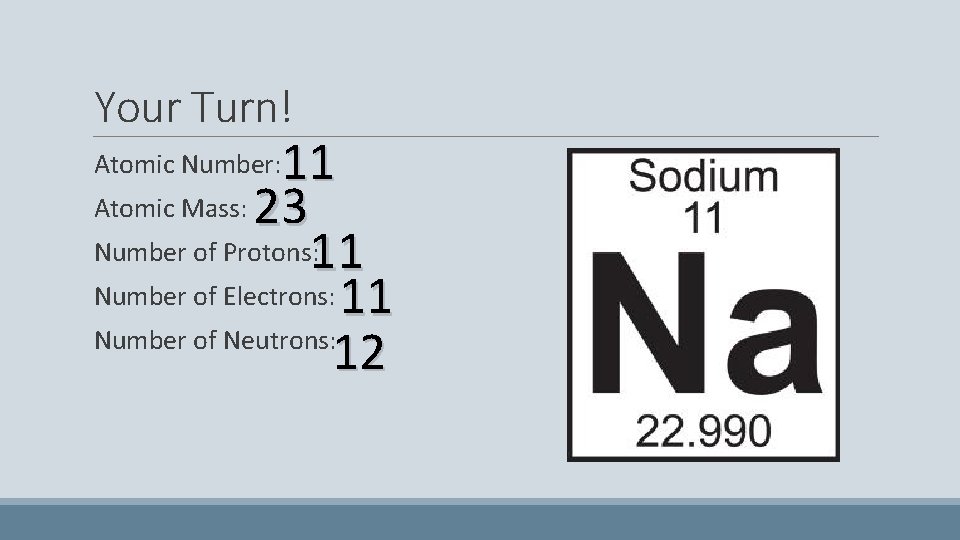
Your Turn! 11 Atomic Mass: 23 Number of Protons: 11 Number of Electrons: 11 Number of Neutrons: 12 Atomic Number:
 Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Isotope abundance formula
Isotope abundance formula Difference between atomic number and atomic mass
Difference between atomic number and atomic mass At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Matterville answer key
Matterville answer key Reference table of atomic radii
Reference table of atomic radii The atoms family song
The atoms family song Matterville worksheet
Matterville worksheet The atoms family atomic math challenge
The atoms family atomic math challenge Matterville answer key
Matterville answer key What are the trends in periodic table
What are the trends in periodic table Ionic radii trends
Ionic radii trends Atomic number vs atomic radius
Atomic number vs atomic radius White matter nervous system
White matter nervous system Primary taste cortex
Primary taste cortex Gray matter and white matter
Gray matter and white matter Pallium telencephalon
Pallium telencephalon Eric’s favourite .......... is science.
Eric’s favourite .......... is science. Are atoms the smallest unit of matter
Are atoms the smallest unit of matter