ASCO 2020 Nouveauts en oncologie thoracique NSCLC translationnel





- Slides: 32

ASCO 2020 Nouveautés en oncologie thoracique

NSCLC translationnel

3518: Early plasma circulating tumor DNA (ct. DNA) changes to predict response to first-line pembrolizumab +/- chemotherapy in non-small cell lung cancer (NSCLC) – Ricciuti B, et al • Study objective – To assess whether early changes in plasma ct. DNA would enable early detection of response to 1 L pembrolizumab ± chemotherapy in patients with NSCLC • Methods – – Treatment-naïve patients with advanced NSCLC were enrolled Blood samples collected on the first day of treatment and before each cycle ct. DNA analyses using In. Vision NGS 38 patients were enrolled • 9 had no detectable ct. DNA • 29 had detectable ct. DNA – 19 received pembrolizumab – 10 received pembrolizumab/pemetrexed/carboplatin Ricciuti B, et al. J Clin Oncol 2020; 38(suppl): Abstr 3518

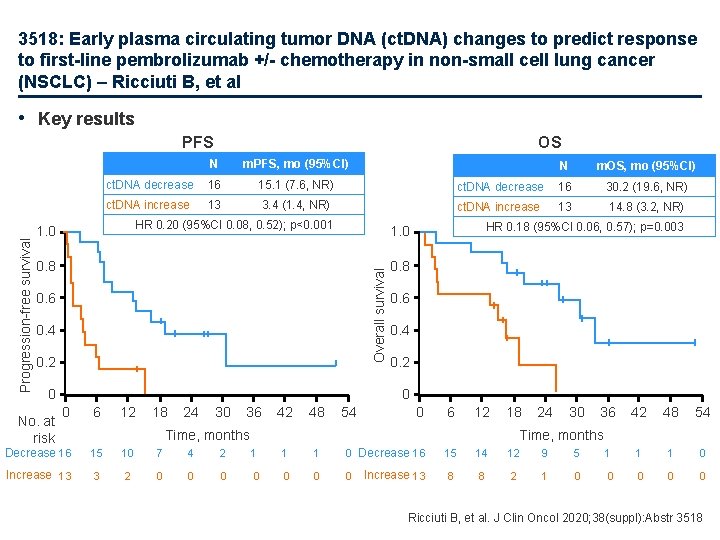
3518: Early plasma circulating tumor DNA (ct. DNA) changes to predict response to first-line pembrolizumab +/- chemotherapy in non-small cell lung cancer (NSCLC) – Ricciuti B, et al • Key results OS N m. PFS, mo (95%CI) ct. DNA decrease 16 15. 1 (7. 6, NR) ct. DNA increase 13 3. 4 (1. 4, NR) HR 0. 20 (95%CI 0. 08, 0. 52); p<0. 001 1. 0 0. 8 0. 6 0. 4 0. 2 0 No. at risk N m. OS, mo (95%CI) ct. DNA decrease 16 30. 2 (19. 6, NR) ct. DNA increase 13 14. 8 (3. 2, NR) HR 0. 18 (95%CI 0. 06, 0. 57); p=0. 003 1. 0 Overall survival Progression-free survival PFS 0. 8 0. 6 0. 4 0. 2 0 0 6 12 18 24 30 36 42 48 54 0 6 12 18 Time, months 24 30 36 42 48 54 Time, months Decrease 16 15 10 7 4 2 1 1 1 0 Decrease 16 15 14 12 9 5 1 1 1 0 Increase 13 3 2 0 0 0 0 Increase 13 8 8 2 1 0 0 0 Ricciuti B, et al. J Clin Oncol 2020; 38(suppl): Abstr 3518

NSCLC stades précoces

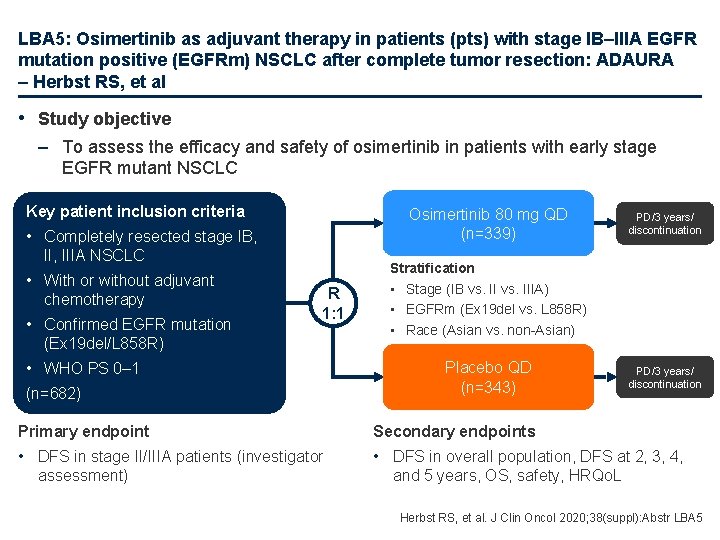
LBA 5: Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA – Herbst RS, et al • Study objective – To assess the efficacy and safety of osimertinib in patients with early stage EGFR mutant NSCLC Key patient inclusion criteria Osimertinib 80 mg QD (n=339) • Completely resected stage IB, IIIA NSCLC • With or without adjuvant chemotherapy • Confirmed EGFR mutation (Ex 19 del/L 858 R) R 1: 1 • WHO PS 0– 1 (n=682) PD/3 years/ discontinuation Stratification • Stage (IB vs. IIIA) • EGFRm (Ex 19 del vs. L 858 R) • Race (Asian vs. non-Asian) Placebo QD (n=343) PD/3 years/ discontinuation Primary endpoint Secondary endpoints • DFS in stage II/IIIA patients (investigator • DFS in overall population, DFS at 2, 3, 4, assessment) and 5 years, OS, safety, HRQo. L Herbst RS, et al. J Clin Oncol 2020; 38(suppl): Abstr LBA 5

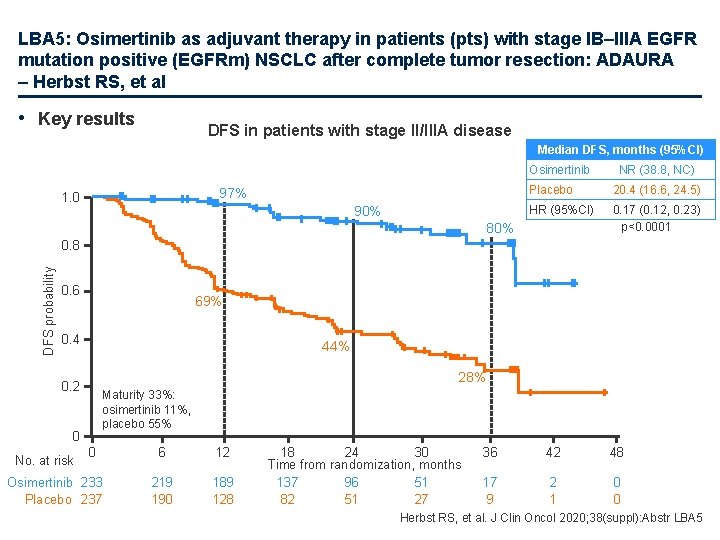
LBA 5: Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA – Herbst RS, et al • Key results DFS in patients with stage II/IIIA disease Median DFS, months (95%CI) Osimertinib 97% 1. 0 90% NR (38. 8, NC) Placebo 20. 4 (16. 6, 24. 5) HR (95%CI) 0. 17 (0. 12, 0. 23) p<0. 0001 80% DFS probability 0. 8 0. 6 69% 0. 4 44% 28% 0. 2 Maturity 33%: osimertinib 11%, placebo 55% 0 No. at risk 0 Osimertinib 233 Placebo 237 6 12 219 190 189 128 18 24 30 Time from randomization, months 137 96 51 82 51 27 36 42 48 17 9 2 1 0 0 Herbst RS, et al. J Clin Oncol 2020; 38(suppl): Abstr LBA 5

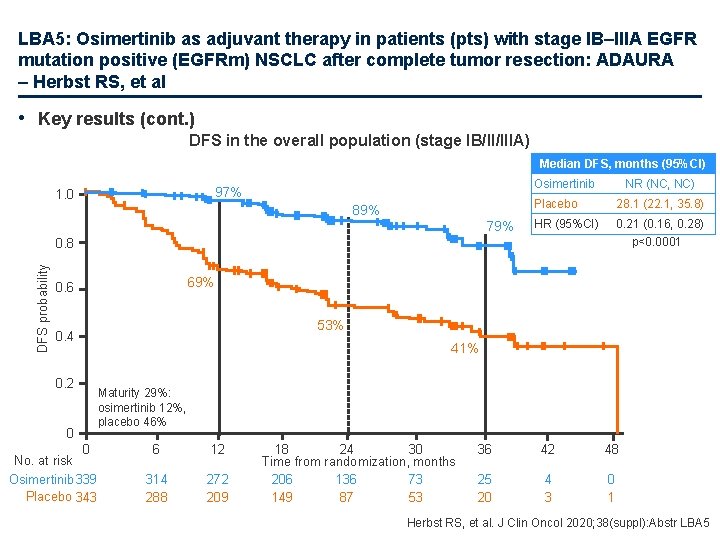
LBA 5: Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA – Herbst RS, et al • Key results (cont. ) DFS in the overall population (stage IB/II/IIIA) Median DFS, months (95%CI) Osimertinib 97% 1. 0 89% 79% NR (NC, NC) Placebo 28. 1 (22. 1, 35. 8) HR (95%CI) 0. 21 (0. 16, 0. 28) p<0. 0001 DFS probability 0. 8 69% 0. 6 53% 0. 4 0. 2 0 41% Maturity 29%: osimertinib 12%, placebo 46% 0 No. at risk Osimertinib 339 Placebo 343 6 12 314 288 272 209 18 24 30 Time from randomization, months 206 136 73 149 87 53 36 42 48 25 20 4 3 0 1 Herbst RS, et al. J Clin Oncol 2020; 38(suppl): Abstr LBA 5

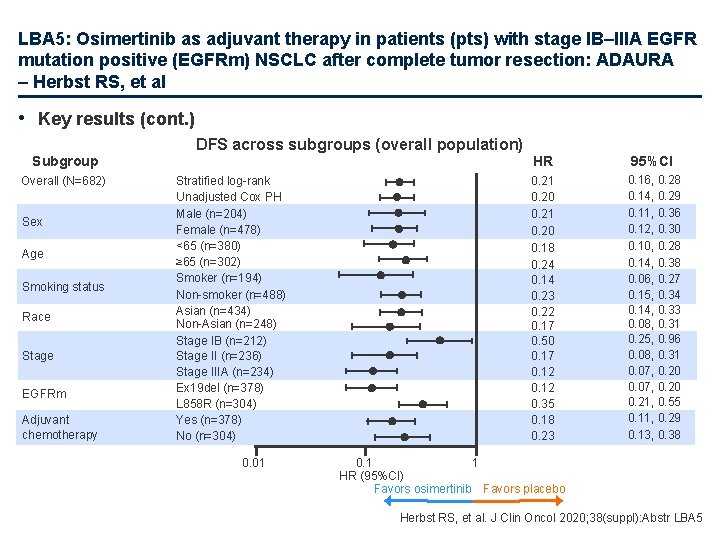
LBA 5: Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA – Herbst RS, et al • Key results (cont. ) DFS across subgroups (overall population) Subgroup Overall (N=682) Sex Age Smoking status Race Stage EGFRm Adjuvant chemotherapy Stratified log-rank Unadjusted Cox PH Male (n=204) Female (n=478) <65 (n=380) ≥ 65 (n=302) Smoker (n=194) Non-smoker (n=488) Asian (n=434) Non-Asian (n=248) Stage IB (n=212) Stage II (n=236) Stage IIIA (n=234) Ex 19 del (n=378) L 858 R (n=304) Yes (n=378) No (n=304) 0. 01 HR 95%CI 0. 21 0. 20 0. 18 0. 24 0. 14 0. 23 0. 22 0. 17 0. 50 0. 17 0. 12 0. 35 0. 18 0. 23 0. 16, 0. 28 0. 14, 0. 29 0. 11, 0. 36 0. 12, 0. 30 0. 10, 0. 28 0. 14, 0. 38 0. 06, 0. 27 0. 15, 0. 34 0. 14, 0. 33 0. 08, 0. 31 0. 25, 0. 96 0. 08, 0. 31 0. 07, 0. 20 0. 21, 0. 55 0. 11, 0. 29 0. 13, 0. 38 0. 1 1 HR (95%CI) Favors osimertinib Favors placebo Herbst RS, et al. J Clin Oncol 2020; 38(suppl): Abstr LBA 5

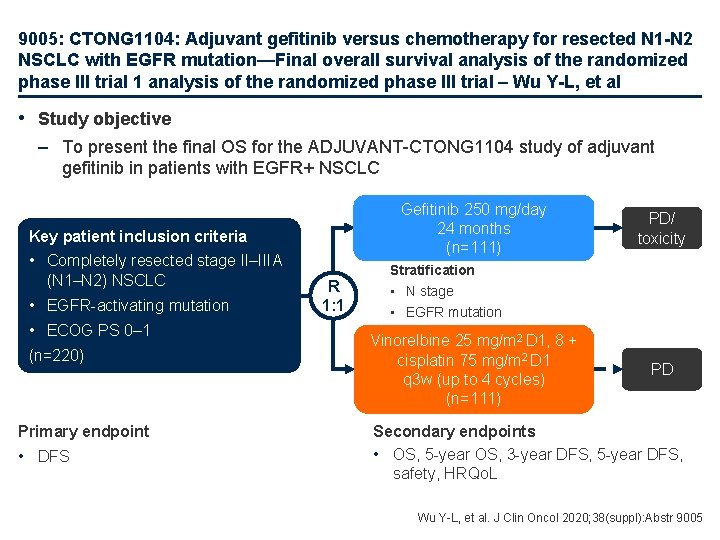
9005: CTONG 1104: Adjuvant gefitinib versus chemotherapy for resected N 1 -N 2 NSCLC with EGFR mutation—Final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial – Wu Y-L, et al • Study objective – To present the final OS for the ADJUVANT-CTONG 1104 study of adjuvant gefitinib in patients with EGFR+ NSCLC Gefitinib 250 mg/day 24 months (n=111) Key patient inclusion criteria • Completely resected stage II–IIIA (N 1–N 2) NSCLC • EGFR-activating mutation • ECOG PS 0– 1 (n=220) Primary endpoint • DFS R 1: 1 PD/ toxicity Stratification • N stage • EGFR mutation Vinorelbine 25 mg/m 2 D 1, 8 + cisplatin 75 mg/m 2 D 1 q 3 w (up to 4 cycles) (n=111) PD Secondary endpoints • OS, 5 -year OS, 3 -year DFS, 5 -year DFS, safety, HRQo. L Wu Y-L, et al. J Clin Oncol 2020; 38(suppl): Abstr 9005

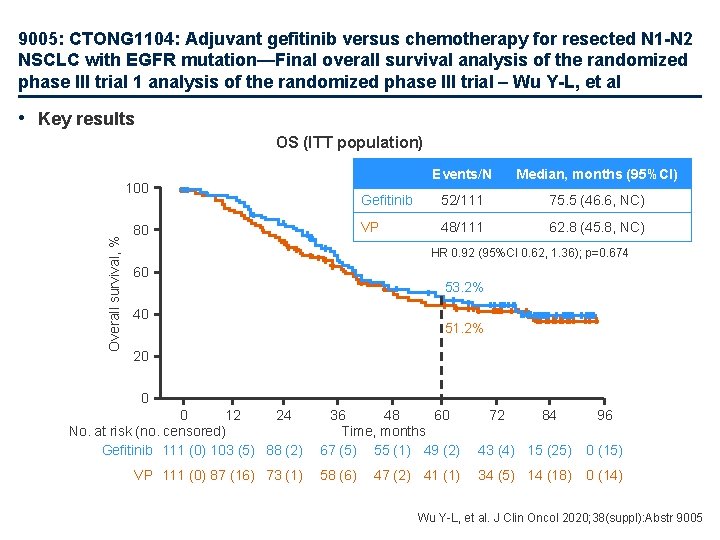
9005: CTONG 1104: Adjuvant gefitinib versus chemotherapy for resected N 1 -N 2 NSCLC with EGFR mutation—Final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial – Wu Y-L, et al • Key results OS (ITT population) Overall survival, % 100 80 Events/N Median, months (95%CI) Gefitinib 52/111 75. 5 (46. 6, NC) VP 48/111 62. 8 (45. 8, NC) HR 0. 92 (95%CI 0. 62, 1. 36); p=0. 674 60 53. 2% 40 51. 2% 20 0 0 12 24 No. at risk (no. censored) Gefitinib 111 (0) 103 (5) 88 (2) VP 111 (0) 87 (16) 73 (1) 36 48 60 Time, months 67 (5) 55 (1) 49 (2) 43 (4) 15 (25) 0 (15) 58 (6) 34 (5) 14 (18) 0 (14) 47 (2) 41 (1) 72 84 96 Wu Y-L, et al. J Clin Oncol 2020; 38(suppl): Abstr 9005

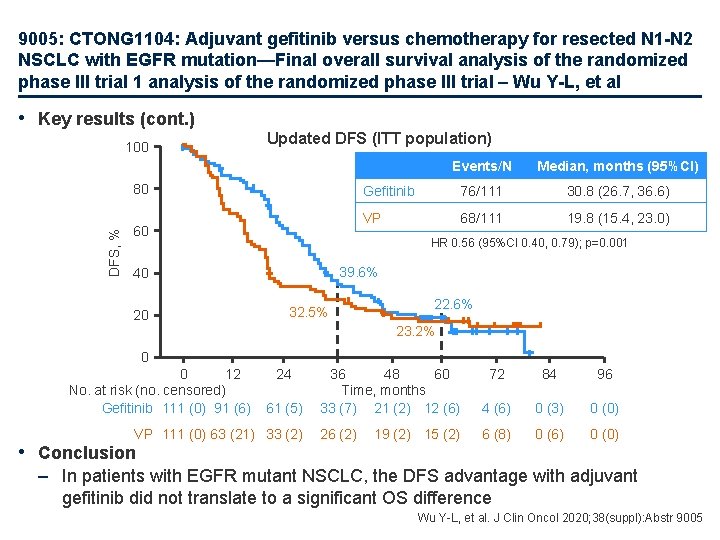
9005: CTONG 1104: Adjuvant gefitinib versus chemotherapy for resected N 1 -N 2 NSCLC with EGFR mutation—Final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial – Wu Y-L, et al • Key results (cont. ) 100 Updated DFS (ITT population) DFS, % 80 60 Median, months (95%CI) Gefitinib 76/111 30. 8 (26. 7, 36. 6) VP 68/111 19. 8 (15. 4, 23. 0) HR 0. 56 (95%CI 0. 40, 0. 79); p=0. 001 39. 6% 40 20 Events/N 32. 5% 22. 6% 23. 2% 0 0 12 No. at risk (no. censored) Gefitinib 111 (0) 91 (6) 24 61 (5) VP 111 (0) 63 (21) 33 (2) • Conclusion 36 48 60 Time, months 33 (7) 21 (2) 12 (6) 72 84 96 4 (6) 0 (3) 0 (0) 26 (2) 6 (8) 0 (6) 0 (0) 19 (2) 15 (2) – In patients with EGFR mutant NSCLC, the DFS advantage with adjuvant gefitinib did not translate to a significant OS difference Wu Y-L, et al. J Clin Oncol 2020; 38(suppl): Abstr 9005

NSCLC stades avancés

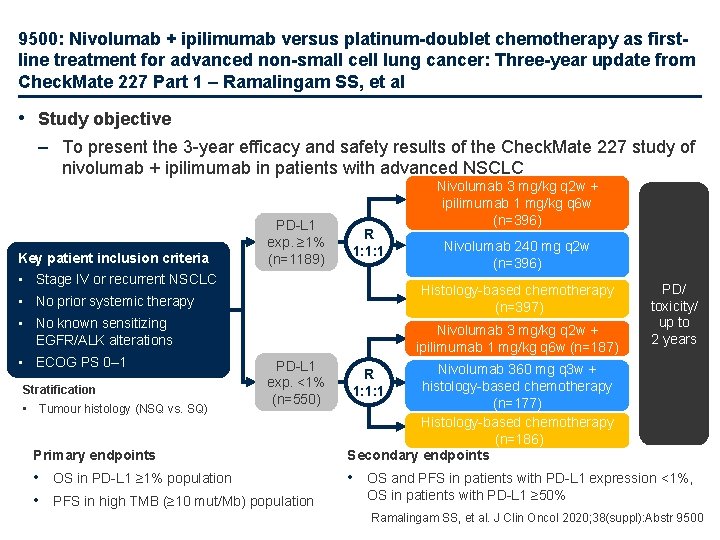
9500: Nivolumab + ipilimumab versus platinum-doublet chemotherapy as firstline treatment for advanced non-small cell lung cancer: Three-year update from Check. Mate 227 Part 1 – Ramalingam SS, et al • Study objective – To present the 3 -year efficacy and safety results of the Check. Mate 227 study of nivolumab + ipilimumab in patients with advanced NSCLC Key patient inclusion criteria PD-L 1 exp. ≥ 1% (n=1189) R 1: 1: 1 • Stage IV or recurrent NSCLC • No known sensitizing EGFR/ALK alterations Stratification • Tumour histology (NSQ vs. SQ) Nivolumab 240 mg q 2 w (n=396) Histology-based chemotherapy (n=397) • No prior systemic therapy • ECOG PS 0– 1 Nivolumab 3 mg/kg q 2 w + ipilimumab 1 mg/kg q 6 w (n=396) Nivolumab 3 mg/kg q 2 w + ipilimumab 1 mg/kg q 6 w (n=187) PD-L 1 exp. <1% (n=550) Primary endpoints • OS in PD-L 1 ≥ 1% population • PFS in high TMB (≥ 10 mut/Mb) population PD/ toxicity/ up to 2 years Nivolumab 360 mg q 3 w + histology-based chemotherapy (n=177) Histology-based chemotherapy (n=186) Secondary endpoints R 1: 1: 1 • OS and PFS in patients with PD-L 1 expression <1%, OS in patients with PD-L 1 ≥ 50% Ramalingam SS, et al. J Clin Oncol 2020; 38(suppl): Abstr 9500

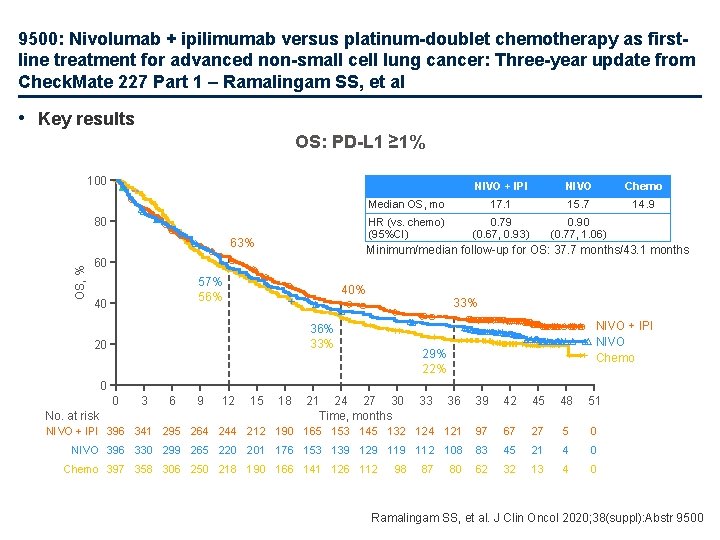
9500: Nivolumab + ipilimumab versus platinum-doublet chemotherapy as firstline treatment for advanced non-small cell lung cancer: Three-year update from Check. Mate 227 Part 1 – Ramalingam SS, et al • Key results OS: PD-L 1 ≥ 1% 100 80 OS, % 63% NIVO Chemo Median OS, mo 17. 1 15. 7 14. 9 HR (vs. chemo) (95%CI) 0. 79 (0. 67, 0. 93) 0. 90 (0. 77, 1. 06) Minimum/median follow-up for OS: 37. 7 months/43. 1 months 60 57% 56% 40 NIVO + IPI 40% 33% 36% 33% 20 NIVO + IPI NIVO Chemo 29% 22% 0 36 39 42 45 48 51 NIVO + IPI 396 341 295 264 244 212 190 165 153 145 132 124 121 97 67 27 5 0 NIVO 396 330 299 265 220 201 176 153 139 129 112 108 83 45 21 4 0 62 32 13 4 0 0 No. at risk 3 6 9 12 15 18 21 24 27 30 Time, months Chemo 397 358 306 250 218 190 166 141 126 112 98 33 87 80 Ramalingam SS, et al. J Clin Oncol 2020; 38(suppl): Abstr 9500

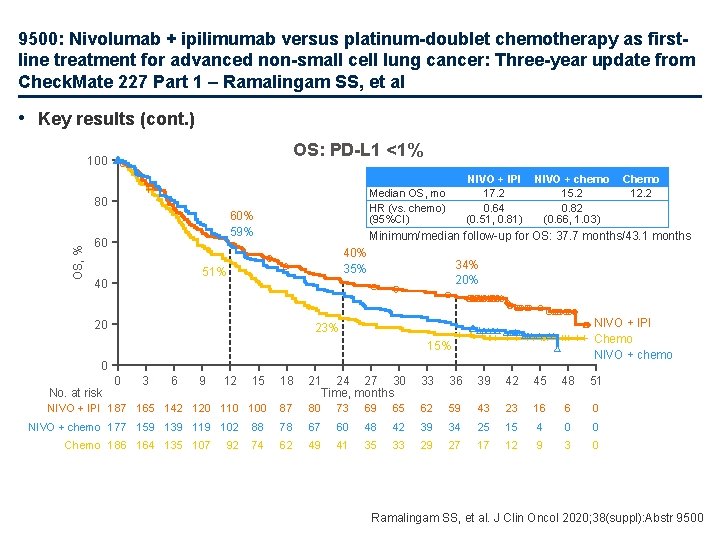
9500: Nivolumab + ipilimumab versus platinum-doublet chemotherapy as firstline treatment for advanced non-small cell lung cancer: Three-year update from Check. Mate 227 Part 1 – Ramalingam SS, et al • Key results (cont. ) OS: PD-L 1 <1% 100 Median OS, mo HR (vs. chemo) (95%CI) OS, % 80 60% 59% 60 NIVO + chemo 15. 2 0. 82 (0. 66, 1. 03) 40% 35% 20 34% 20% NIVO + IPI Chemo NIVO + chemo 23% 15% 0 15 18 21 24 27 30 Time, months 33 36 39 42 45 48 51 NIVO + IPI 187 165 142 120 110 100 87 80 73 69 65 62 59 43 23 16 6 0 88 78 67 60 48 42 39 34 25 15 4 0 0 74 62 49 41 35 33 29 27 17 12 9 3 0 No. at risk 0 3 6 9 12 NIVO + chemo 177 159 139 119 102 Chemo 186 164 135 107 92 Chemo 12. 2 Minimum/median follow-up for OS: 37. 7 months/43. 1 months 51% 40 NIVO + IPI 17. 2 0. 64 (0. 51, 0. 81) Ramalingam SS, et al. J Clin Oncol 2020; 38(suppl): Abstr 9500

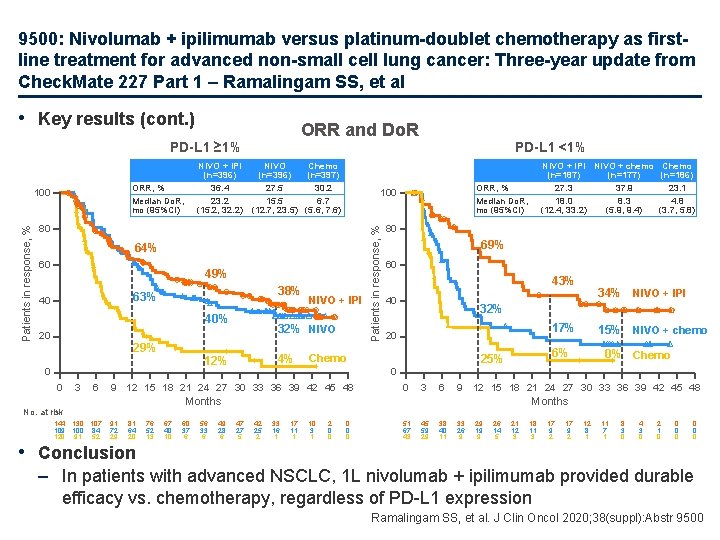
9500: Nivolumab + ipilimumab versus platinum-doublet chemotherapy as firstline treatment for advanced non-small cell lung cancer: Three-year update from Check. Mate 227 Part 1 – Ramalingam SS, et al • Key results (cont. ) ORR and Do. R PD-L 1 ≥ 1% 80 64% 60 49% 38% 63% 40 40% 20 29% 0 3 6 4% 80 69% 60 43% 40 17% 20 81 64 20 • Conclusion 76 52 13 67 40 10 60 37 6 56 33 6 49 28 6 15% NIVO + chemo 6% 25% 0% Chemo 0 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 Months 91 72 29 34% NIVO + IPI 32% Chemo 9 12 15 18 21 24 27 30 33 36 39 42 45 48 No. at risk 144 130 107 109 100 84 120 91 52 32% NIVO 12% 0 NIVO + IPI NIVO + chemo Chemo (n=187) (n=177) (n=186) 27. 3 37. 9 23. 1 18. 0 8. 3 4. 8 (12. 4, 33. 2) (5. 9, 9. 4) (3. 7, 5. 8) ORR, % Median Do. R, mo (95%CI) 100 Patients in response, % NIVO + IPI NIVO Chemo (n=396) (n=397) 36. 4 27. 5 30. 2 23. 2 15. 5 6. 7 (15. 2, 32. 2) (12. 7, 23. 5) (5. 6, 7. 6) ORR, % Median Do. R, mo (95%CI) 100 PD-L 1 <1% Months 47 27 5 42 25 2 33 16 1 17 11 1 10 3 1 2 0 0 0 51 67 43 45 59 29 38 40 11 33 26 9 29 19 9 26 14 5 21 12 3 18 11 3 17 9 2 12 8 1 11 7 1 8 3 0 4 3 0 2 1 0 0 0 0 – In patients with advanced NSCLC, 1 L nivolumab + ipilimumab provided durable efficacy vs. chemotherapy, regardless of PD-L 1 expression Ramalingam SS, et al. J Clin Oncol 2020; 38(suppl): Abstr 9500

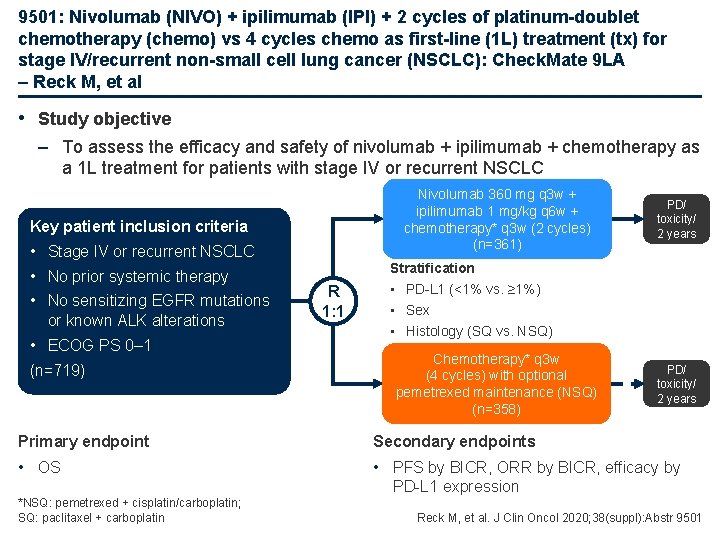
9501: Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1 L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): Check. Mate 9 LA – Reck M, et al • Study objective – To assess the efficacy and safety of nivolumab + ipilimumab + chemotherapy as a 1 L treatment for patients with stage IV or recurrent NSCLC Nivolumab 360 mg q 3 w + ipilimumab 1 mg/kg q 6 w + chemotherapy* q 3 w (2 cycles) (n=361) Key patient inclusion criteria • Stage IV or recurrent NSCLC • No prior systemic therapy • No sensitizing EGFR mutations or known ALK alterations • ECOG PS 0– 1 (n=719) R 1: 1 PD/ toxicity/ 2 years Stratification • PD-L 1 (<1% vs. ≥ 1%) • Sex • Histology (SQ vs. NSQ) Chemotherapy* q 3 w (4 cycles) with optional pemetrexed maintenance (NSQ) (n=358) PD/ toxicity/ 2 years Primary endpoint Secondary endpoints • OS • PFS by BICR, ORR by BICR, efficacy by PD-L 1 expression *NSQ: pemetrexed + cisplatin/carboplatin; SQ: paclitaxel + carboplatin Reck M, et al. J Clin Oncol 2020; 38(suppl): Abstr 9501

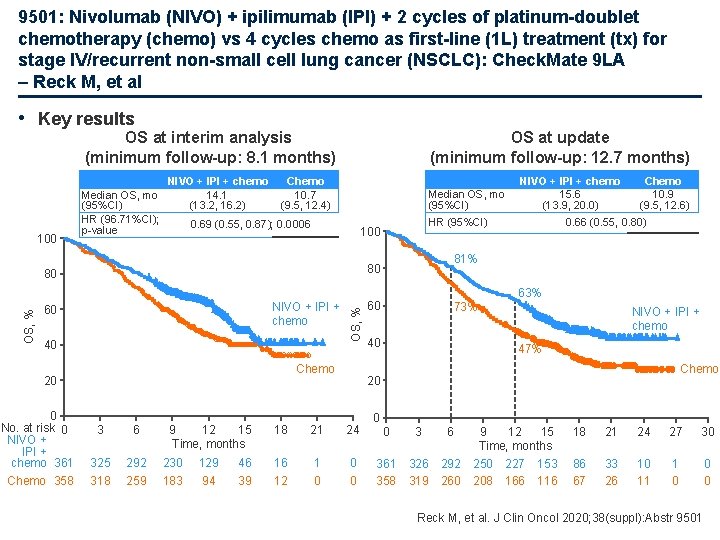
9501: Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1 L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): Check. Mate 9 LA – Reck M, et al • Key results OS at interim analysis (minimum follow-up: 8. 1 months) 100 Median OS, mo (95%CI) HR (96. 71%CI); p-value NIVO + IPI + chemo 14. 1 (13. 2, 16. 2) OS at update (minimum follow-up: 12. 7 months) Chemo 10. 7 (9. 5, 12. 4) Median OS, mo (95%CI) 0. 69 (0. 55, 0. 87); 0. 0006 HR (95%CI) 100 Chemo 10. 9 (9. 5, 12. 6) 0. 66 (0. 55, 0. 80) 81% 80 80 NIVO + IPI + chemo 15. 6 (13. 9, 20. 0) 60 40 OS, % 63% NIVO + IPI + chemo Chemo 20 0 No. at risk 0 NIVO + IPI + chemo 361 3 6 325 292 230 129 Chemo 358 318 259 183 94 9 12 15 Time, months 60 73% 40 NIVO + IPI + chemo 47% Chemo 20 18 21 24 46 16 1 0 39 12 0 0 3 6 361 358 326 319 292 260 9 12 15 Time, months 250 227 153 208 166 116 18 21 24 27 30 86 67 33 26 10 11 1 0 0 0 Reck M, et al. J Clin Oncol 2020; 38(suppl): Abstr 9501

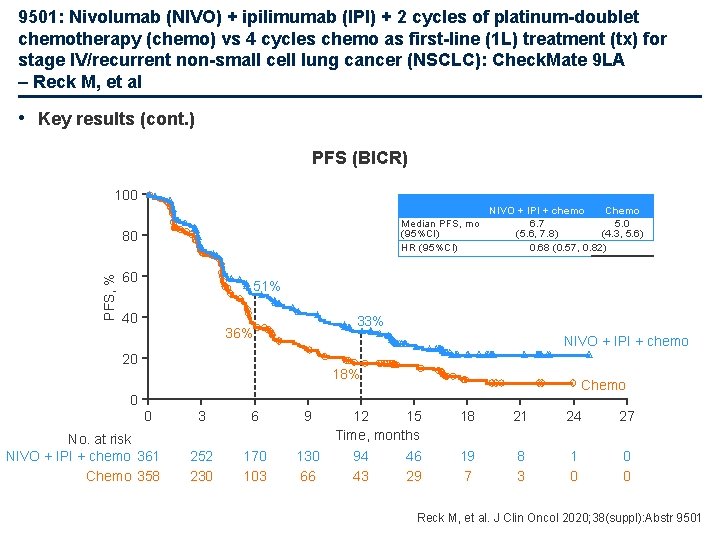
9501: Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1 L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): Check. Mate 9 LA – Reck M, et al • Key results (cont. ) PFS (BICR) 100 Median PFS, mo (95%CI) HR (95%CI) PFS, % 80 60 NIVO + IPI + chemo Chemo 6. 7 5. 0 (5. 6, 7. 8) (4. 3, 5. 6) 0. 68 (0. 57, 0. 82) 51% 40 33% 36% NIVO + IPI + chemo 20 18% Chemo 0 0 No. at risk NIVO + IPI + chemo 361 Chemo 358 3 6 9 252 230 170 103 130 66 12 15 Time, months 94 46 43 29 18 21 24 27 19 7 8 3 1 0 0 0 Reck M, et al. J Clin Oncol 2020; 38(suppl): Abstr 9501

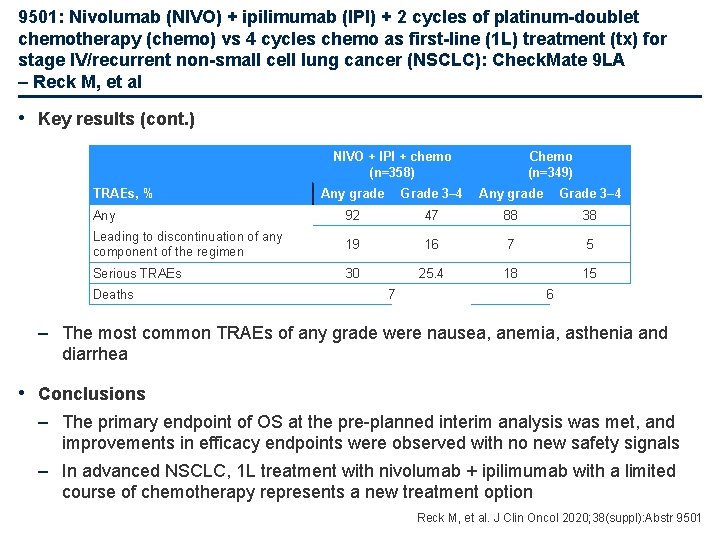
9501: Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1 L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): Check. Mate 9 LA – Reck M, et al • Key results (cont. ) NIVO + IPI + chemo (n=358) TRAEs, % Chemo (n=349) Any grade Grade 3– 4 Any 92 47 88 38 Leading to discontinuation of any component of the regimen 19 16 7 5 Serious TRAEs 30 25. 4 18 15 Deaths 7 6 – The most common TRAEs of any grade were nausea, anemia, asthenia and diarrhea • Conclusions – The primary endpoint of OS at the pre-planned interim analysis was met, and improvements in efficacy endpoints were observed with no new safety signals – In advanced NSCLC, 1 L treatment with nivolumab + ipilimumab with a limited course of chemotherapy represents a new treatment option Reck M, et al. J Clin Oncol 2020; 38(suppl): Abstr 9501

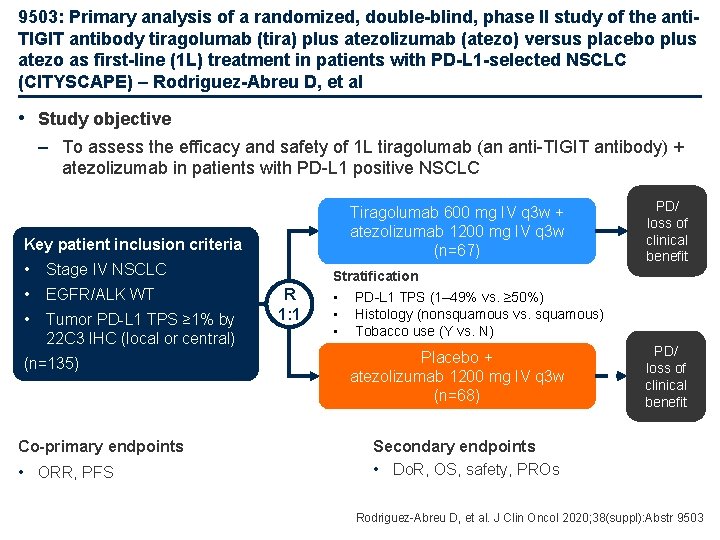
9503: Primary analysis of a randomized, double-blind, phase II study of the anti. TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1 L) treatment in patients with PD-L 1 -selected NSCLC (CITYSCAPE) – Rodriguez-Abreu D, et al • Study objective – To assess the efficacy and safety of 1 L tiragolumab (an anti-TIGIT antibody) + atezolizumab in patients with PD-L 1 positive NSCLC Tiragolumab 600 mg IV q 3 w + atezolizumab 1200 mg IV q 3 w (n=67) Key patient inclusion criteria • Stage IV NSCLC • EGFR/ALK WT • Tumor PD-L 1 TPS ≥ 1% by 22 C 3 IHC (local or central) (n=135) Co-primary endpoints • ORR, PFS R 1: 1 PD/ loss of clinical benefit Stratification • PD-L 1 TPS (1– 49% vs. ≥ 50%) • Histology (nonsquamous vs. squamous) • Tobacco use (Y vs. N) Placebo + atezolizumab 1200 mg IV q 3 w (n=68) PD/ loss of clinical benefit Secondary endpoints • Do. R, OS, safety, PROs Rodriguez-Abreu D, et al. J Clin Oncol 2020; 38(suppl): Abstr 9503

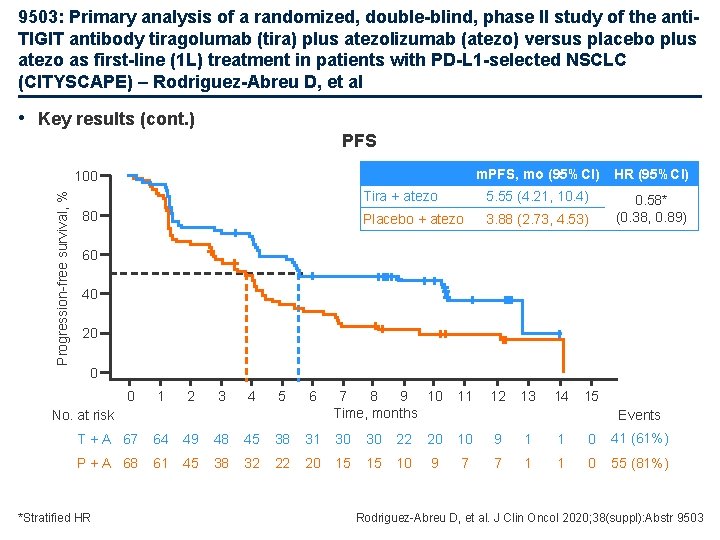
9503: Primary analysis of a randomized, double-blind, phase II study of the anti. TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1 L) treatment in patients with PD-L 1 -selected NSCLC (CITYSCAPE) – Rodriguez-Abreu D, et al • Key results (cont. ) PFS m. PFS, mo (95%CI) HR (95%CI) Tira + atezo 5. 55 (4. 21, 10. 4) Placebo + atezo 3. 88 (2. 73, 4. 53) 0. 58* (0. 38, 0. 89) Progression-free survival, % 100 80 60 40 20 0 0 1 2 3 4 5 6 No. at risk 7 8 9 10 Time, months 11 12 13 14 15 Events T + A 67 64 49 48 45 38 31 30 30 22 20 10 9 1 1 0 41 (61%) P + A 68 61 45 38 32 22 20 15 15 10 9 7 7 1 1 0 55 (81%) *Stratified HR Rodriguez-Abreu D, et al. J Clin Oncol 2020; 38(suppl): Abstr 9503

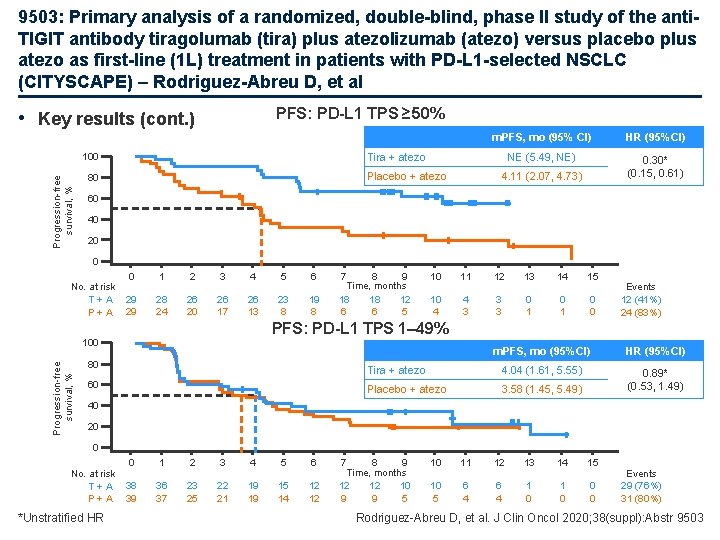
9503: Primary analysis of a randomized, double-blind, phase II study of the anti. TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1 L) treatment in patients with PD-L 1 -selected NSCLC (CITYSCAPE) – Rodriguez-Abreu D, et al PFS: PD-L 1 TPS ≥ 50% • Key results (cont. ) HR (95%CI) NE (5. 49, NE) 0. 30* (0. 15, 0. 61) Tira + atezo 100 Progression-free survival, % m. PFS, mo (95% CI) Placebo + atezo 80 4. 11 (2. 07, 4. 73) 60 40 20 0 No. at risk T+A P+A 0 1 2 3 4 5 6 29 29 28 24 26 20 26 17 26 13 23 8 19 8 7 8 9 Time, months 18 18 12 6 6 5 10 11 12 13 14 15 10 4 4 3 3 3 0 1 0 0 Events 12 (41%) 24 (83%) PFS: PD-L 1 TPS 1– 49% Progression-free survival, % 100 80 60 m. PFS, mo (95%CI) HR (95%CI) Tira + atezo 4. 04 (1. 61, 5. 55) Placebo + atezo 3. 58 (1. 45, 5. 49) 0. 89* (0. 53, 1. 49) 40 20 0 No. at risk T+A P+A *Unstratified HR 0 1 2 3 4 5 6 38 39 36 37 23 25 22 21 19 19 15 14 12 12 7 8 9 Time, months 12 12 10 9 9 5 10 11 12 13 14 15 10 5 6 4 1 0 0 0 Events 29 (76%) 31 (80%) Rodriguez-Abreu D, et al. J Clin Oncol 2020; 38(suppl): Abstr 9503

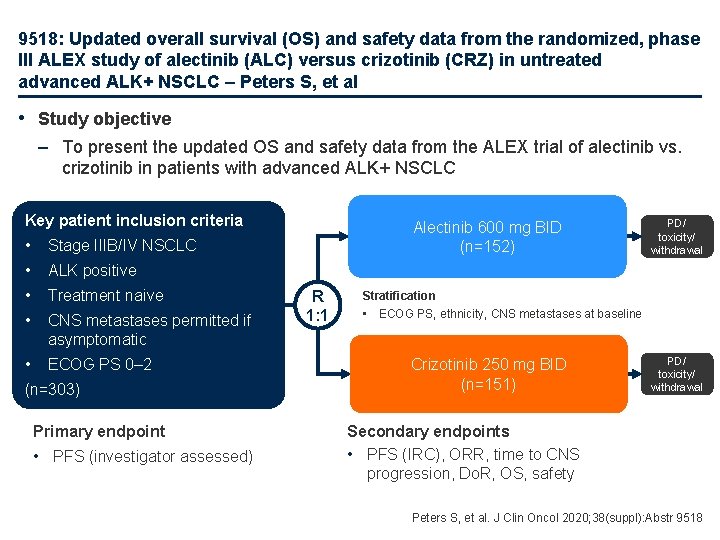
9518: Updated overall survival (OS) and safety data from the randomized, phase III ALEX study of alectinib (ALC) versus crizotinib (CRZ) in untreated advanced ALK+ NSCLC – Peters S, et al • Study objective – To present the updated OS and safety data from the ALEX trial of alectinib vs. crizotinib in patients with advanced ALK+ NSCLC Key patient inclusion criteria • Stage IIIB/IV NSCLC • ALK positive • Treatment naive • CNS metastases permitted if asymptomatic • ECOG PS 0– 2 (n=303) Primary endpoint • PFS (investigator assessed) Alectinib 600 mg BID (n=152) R 1: 1 PD/ toxicity/ withdrawal Stratification • ECOG PS, ethnicity, CNS metastases at baseline Crizotinib 250 mg BID (n=151) PD/ toxicity/ withdrawal Secondary endpoints • PFS (IRC), ORR, time to CNS progression, Do. R, OS, safety Peters S, et al. J Clin Oncol 2020; 38(suppl): Abstr 9518

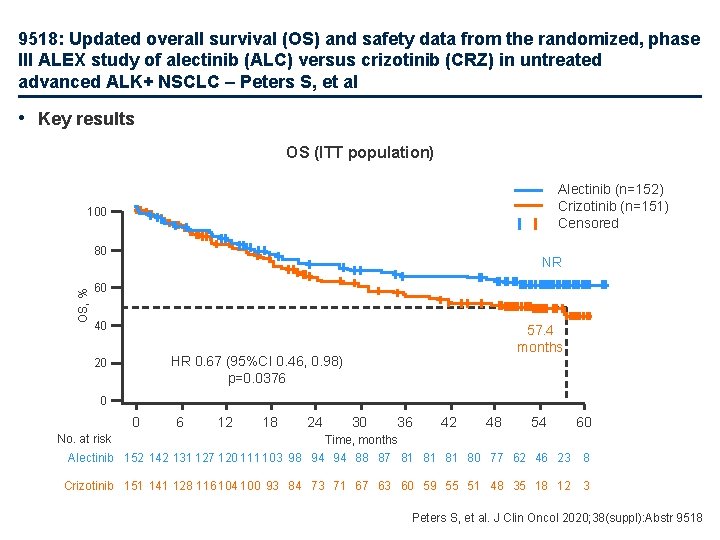
9518: Updated overall survival (OS) and safety data from the randomized, phase III ALEX study of alectinib (ALC) versus crizotinib (CRZ) in untreated advanced ALK+ NSCLC – Peters S, et al • Key results OS (ITT population) Alectinib (n=152) Crizotinib (n=151) Censored 100 OS, % 80 NR 60 40 57. 4 months HR 0. 67 (95%CI 0. 46, 0. 98) p=0. 0376 20 0 0 6 12 18 24 30 36 42 48 54 60 No. at risk Time, months Alectinib 152 142 131 127 120 111 103 98 94 94 88 87 81 81 81 80 77 62 46 23 8 Crizotinib 151 141 128 116 104 100 93 84 73 71 67 63 60 59 55 51 48 35 18 12 3 Peters S, et al. J Clin Oncol 2020; 38(suppl): Abstr 9518

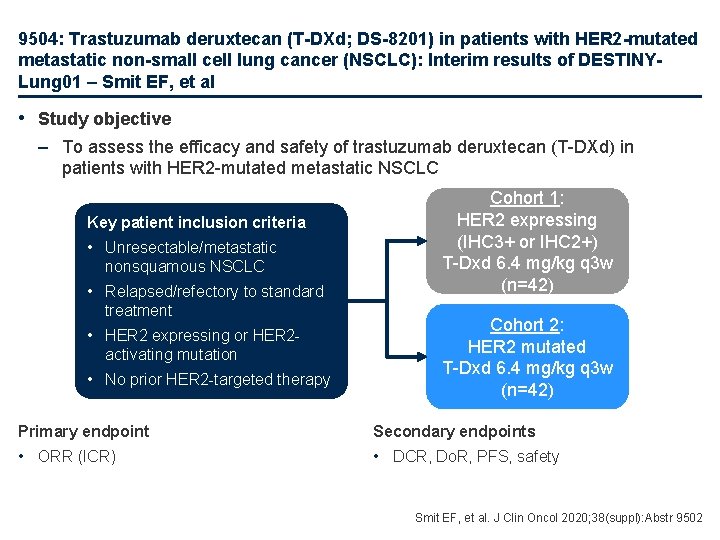
9504: Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER 2 -mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINYLung 01 – Smit EF, et al • Study objective – To assess the efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients with HER 2 -mutated metastatic NSCLC Key patient inclusion criteria • Unresectable/metastatic nonsquamous NSCLC • Relapsed/refectory to standard treatment • HER 2 expressing or HER 2 activating mutation • No prior HER 2 -targeted therapy Cohort 1: HER 2 expressing (IHC 3+ or IHC 2+) T-Dxd 6. 4 mg/kg q 3 w (n=42) Cohort 2: HER 2 mutated T-Dxd 6. 4 mg/kg q 3 w (n=42) Primary endpoint Secondary endpoints • ORR (ICR) • DCR, Do. R, PFS, safety Smit EF, et al. J Clin Oncol 2020; 38(suppl): Abstr 9502

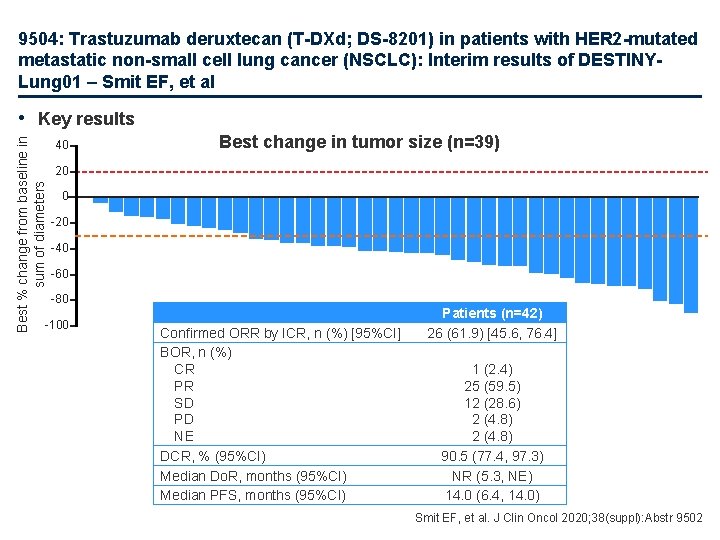
9504: Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER 2 -mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINYLung 01 – Smit EF, et al Best % change from baseline in sum of diameters • Key results 40 Best change in tumor size (n=39) 20 0 -20 -40 -60 -80 -100 Confirmed ORR by ICR, n (%) [95%CI] BOR, n (%) CR PR SD PD NE DCR, % (95%CI) Median Do. R, months (95%CI) Median PFS, months (95%CI) Patients (n=42) 26 (61. 9) [45. 6, 76. 4] 1 (2. 4) 25 (59. 5) 12 (28. 6) 2 (4. 8) 90. 5 (77. 4, 97. 3) NR (5. 3, NE) 14. 0 (6. 4, 14. 0) Smit EF, et al. J Clin Oncol 2020; 38(suppl): Abstr 9502

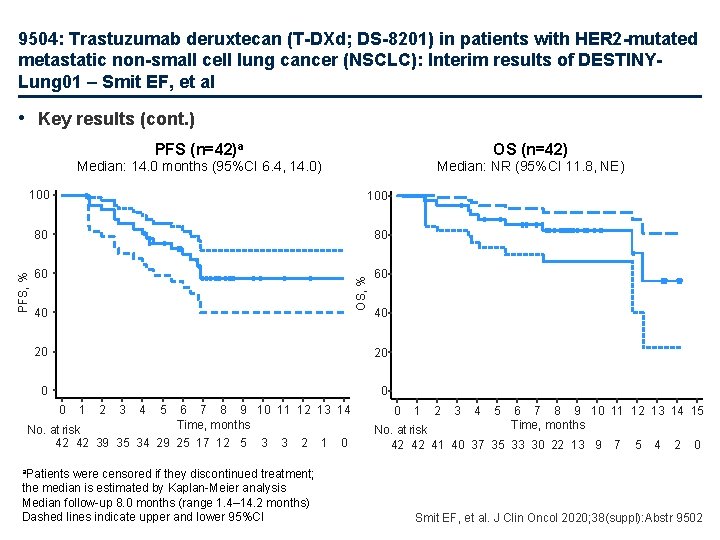
9504: Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER 2 -mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINYLung 01 – Smit EF, et al • Key results (cont. ) PFS (n=42)a OS (n=42) Median: 14. 0 months (95%CI 6. 4, 14. 0) Median: NR (95%CI 11. 8, NE) 80 80 60 60 OS, % 100 PFS, % 100 40 40 20 20 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Time, months No. at risk 42 42 39 35 34 29 25 17 12 5 3 3 2 were censored if they discontinued treatment; the median is estimated by Kaplan-Meier analysis Median follow-up 8. 0 months (range 1. 4– 14. 2 months) Dashed lines indicate upper and lower 95%CI 1 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Time, months No. at risk 42 42 41 40 37 35 33 30 22 13 9 7 5 4 2 0 a. Patients Smit EF, et al. J Clin Oncol 2020; 38(suppl): Abstr 9502

SCLC stades étendus

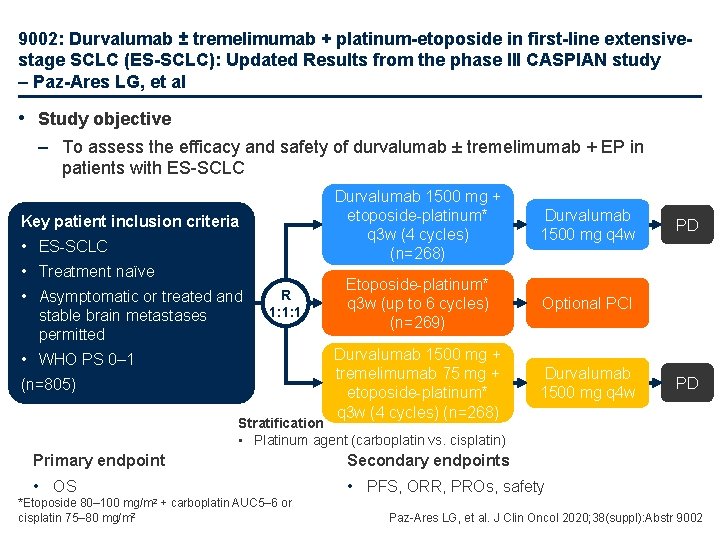
9002: Durvalumab ± tremelimumab + platinum-etoposide in first-line extensivestage SCLC (ES-SCLC): Updated Results from the phase III CASPIAN study – Paz-Ares LG, et al • Study objective – To assess the efficacy and safety of durvalumab ± tremelimumab + EP in patients with ES-SCLC Key patient inclusion criteria • ES-SCLC • Treatment naïve • Asymptomatic or treated and stable brain metastases permitted R 1: 1: 1 • WHO PS 0– 1 (n=805) Durvalumab 1500 mg + etoposide-platinum* q 3 w (4 cycles) (n=268) Durvalumab 1500 mg q 4 w Etoposide-platinum* q 3 w (up to 6 cycles) (n=269) Optional PCI Durvalumab 1500 mg + tremelimumab 75 mg + etoposide-platinum* q 3 w (4 cycles) (n=268) Durvalumab 1500 mg q 4 w PD PD Stratification • Platinum agent (carboplatin vs. cisplatin) Primary endpoint Secondary endpoints • OS • PFS, ORR, PROs, safety mg/m 2 *Etoposide 80– 100 cisplatin 75– 80 mg/m 2 + carboplatin AUC 5– 6 or Paz-Ares LG, et al. J Clin Oncol 2020; 38(suppl): Abstr 9002

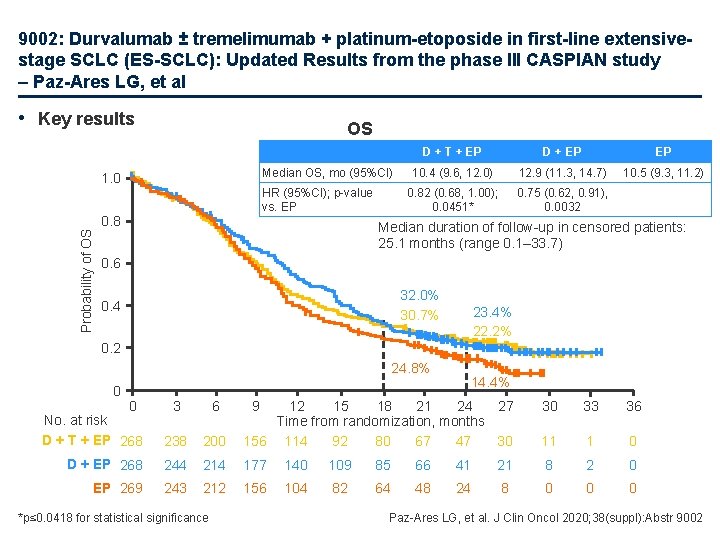
9002: Durvalumab ± tremelimumab + platinum-etoposide in first-line extensivestage SCLC (ES-SCLC): Updated Results from the phase III CASPIAN study – Paz-Ares LG, et al • Key results OS Median OS, mo (95%CI) Probability of OS 1. 0 HR (95%CI); p-value vs. EP 0. 8 D + T + EP D + EP EP 10. 4 (9. 6, 12. 0) 12. 9 (11. 3, 14. 7) 10. 5 (9. 3, 11. 2) 0. 82 (0. 68, 1. 00); 0. 0451* 0. 75 (0. 62, 0. 91), 0. 0032 Median duration of follow-up in censored patients: 25. 1 months (range 0. 1– 33. 7) 0. 6 32. 0% 30. 7% 0. 4 23. 4% 22. 2% 0. 2 24. 8% 0 0 No. at risk D + T + EP 268 3 6 238 D + EP 268 EP 269 14. 4% 30 33 36 200 12 15 18 21 24 27 Time from randomization, months 156 114 92 80 67 47 30 11 1 0 244 214 177 140 109 85 66 41 21 8 2 0 243 212 156 104 82 64 48 24 8 0 0 0 *p≤ 0. 0418 for statistical significance 9 Paz-Ares LG, et al. J Clin Oncol 2020; 38(suppl): Abstr 9002
 Translationnel
Translationnel Psycho oncologie
Psycho oncologie Institut national d'oncologie rabat
Institut national d'oncologie rabat Adjuvant nsclc
Adjuvant nsclc Nsclc
Nsclc Adjuvant nsclc
Adjuvant nsclc 2017 asco oncology practice conference
2017 asco oncology practice conference ¿cuál es tu restaurante favorito?
¿cuál es tu restaurante favorito? Joanne chien
Joanne chien Coi rubber
Coi rubber Diferencia entre centinela y atalaya
Diferencia entre centinela y atalaya Asco gu san francisco
Asco gu san francisco Gunter von minckwitz
Gunter von minckwitz Sna anatomie
Sna anatomie Champs pulmonaires clairs
Champs pulmonaires clairs Intra thoracique
Intra thoracique Index cardio thoracique
Index cardio thoracique Drain thoracique surveillance infirmière
Drain thoracique surveillance infirmière Pinire
Pinire Signe de l'iceberg radio
Signe de l'iceberg radio Drain thoracique thopaz
Drain thoracique thopaz Articulation scapulo thoracique
Articulation scapulo thoracique Toux moniliforme definition
Toux moniliforme definition Le canal thoracique
Le canal thoracique Calcul index cardio thoracique
Calcul index cardio thoracique Pose d'un drain thoracique
Pose d'un drain thoracique 2020 revised curriculum and assessment plans
2020 revised curriculum and assessment plans Science olympiad astronomy
Science olympiad astronomy Nomenclatorul documentatiei scolare 2020-2021
Nomenclatorul documentatiei scolare 2020-2021 Solucionario libro de historia 2 medio 2020
Solucionario libro de historia 2 medio 2020 Pet reading part 6 exercises
Pet reading part 6 exercises Irpwm 2020
Irpwm 2020 Sap sms 365
Sap sms 365