2017 ASCO Annual Meeting 2 6 June 2017







- Slides: 49

2017 ASCO Annual Meeting 2– 6 June 2017 | Chicago, USA Supported by Eli Lilly and Company has not influenced the content of this publication

Letter from Prof Jean Yves-Blay Dear Colleagues It is my pleasure to present this WSN slide set which has been designed to highlight and summarise key findings in sarcoma from the major congresses in 2017. This slide set specifically focuses on the 53 rd Annual Meeting of the American Society of Clinical Oncology. The area of clinical research in oncology is a challenging and ever changing environment. Within this environment we all value access to scientific data and research that helps to educate and inspire further advancements in our roles as scientists, clinicians and educators. I hope you find this review of the latest developments in sarcoma of benefit to you in your practice. I would like to thank our WSN members Drs Piotr Rutkowski and Claudia Valverde for their roles as Editors – for prioritising abstracts and reviewing slide content. The slide set you see before you would not be possible without their commitment and hard work. And finally, we are also very grateful to Lilly Oncology for their financial, administrial and logistical support in the realisation of this complex yet rewarding activity. Yours sincerely, Jean Yves-Blay WSN Chairman of the Board

WSN Medical Oncology Slide Deck Editors 2017 Piotr Rutkowski Maria Sklodowska-Curie Institute – Oncology Center, Warsaw, Poland Claudia Valverde Vall d’Hebron University Hospital, Barcelona, Spain

Contents • Biomarkers and screening 5 • Locally advanced sarcoma 18 • Advanced/metastatic sarcoma: chemotherapy, targeted therapy and immunotherapy 25

Biomarkers and screening

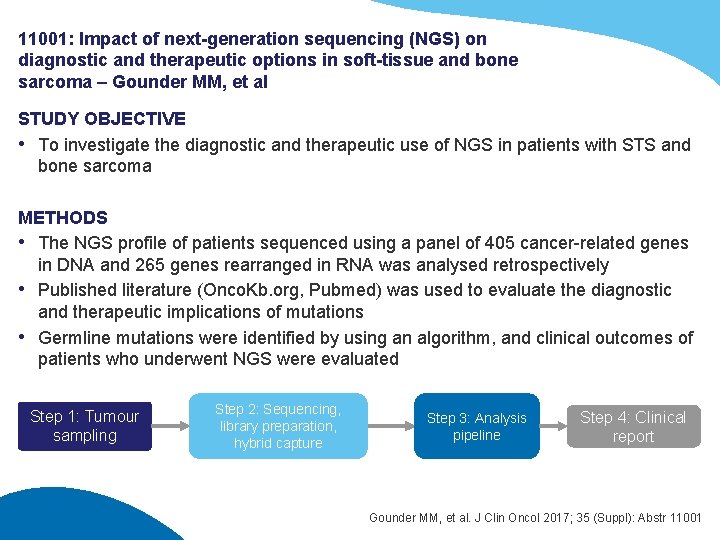
11001: Impact of next-generation sequencing (NGS) on diagnostic and therapeutic options in soft-tissue and bone sarcoma – Gounder MM, et al STUDY OBJECTIVE • To investigate the diagnostic and therapeutic use of NGS in patients with STS and bone sarcoma METHODS • The NGS profile of patients sequenced using a panel of 405 cancer-related genes in DNA and 265 genes rearranged in RNA was analysed retrospectively • Published literature (Onco. Kb. org, Pubmed) was used to evaluate the diagnostic and therapeutic implications of mutations • Germline mutations were identified by using an algorithm, and clinical outcomes of patients who underwent NGS were evaluated Step 1: Tumour sampling Step 2: Sequencing, library preparation, hybrid capture Step 3: Analysis pipeline Step 4: Clinical report Gounder MM, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11001

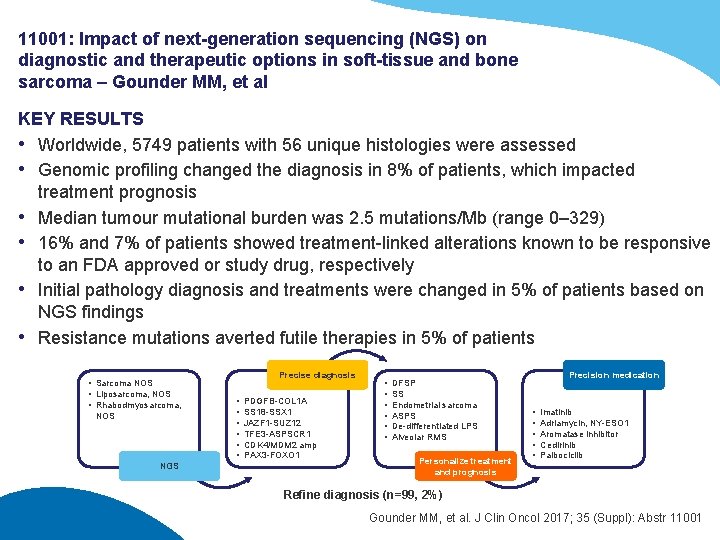
11001: Impact of next-generation sequencing (NGS) on diagnostic and therapeutic options in soft-tissue and bone sarcoma – Gounder MM, et al KEY RESULTS • Worldwide, 5749 patients with 56 unique histologies were assessed • Genomic profiling changed the diagnosis in 8% of patients, which impacted treatment prognosis • Median tumour mutational burden was 2. 5 mutations/Mb (range 0– 329) • 16% and 7% of patients showed treatment-linked alterations known to be responsive to an FDA approved or study drug, respectively • Initial pathology diagnosis and treatments were changed in 5% of patients based on NGS findings • Resistance mutations averted futile therapies in 5% of patients • Sarcoma NOS • Liposarcoma, NOS • Rhabodmyosarcoma, NOS NGS Precise diagnosis • • • PDGFB-COL 1 A SS 18 -SSX 1 JAZF 1 -SUZ 12 TFE 3 -ASPSCR 1 CDK 4/MDM 2 amp PAX 3 -FOXO 1 • • • DFSP SS Endometrial sarcoma ASPS De-differentiated LPS Alveolar RMS Personalize treatment and prognosis Precision medication • • • Imatinib Adriamycin, NY-ESO 1 Aromatase inhibitor Cedirinib Palbociclib Refine diagnosis (n=99, 2%) Gounder MM, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11001

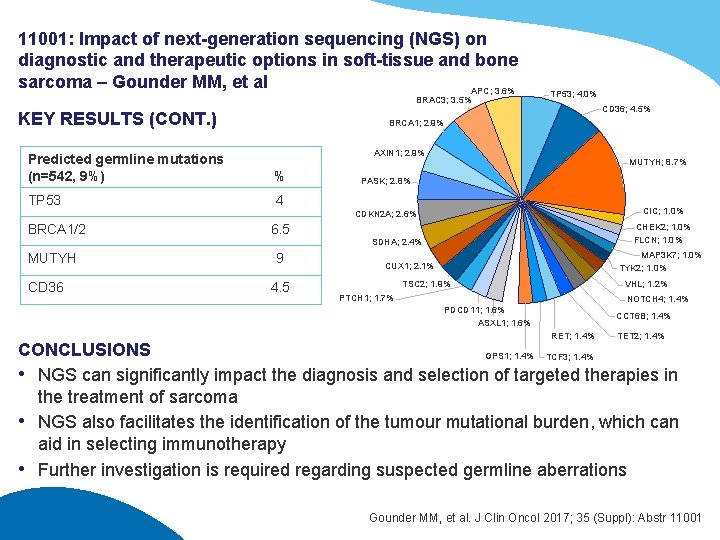
11001: Impact of next-generation sequencing (NGS) on diagnostic and therapeutic options in soft-tissue and bone sarcoma – Gounder MM, et al APC; 3. 6% BRAC 3; 3. 5% KEY RESULTS (CONT. ) TP 53; 4. 0% CD 36; 4. 5% BRCA 1; 2. 9% AXIN 1; 2. 9% Predicted germline mutations (n=542, 9%) % TP 53 4 MUTYH; 8. 7% PASK; 2. 8% CIC; 1. 0% CDKN 2 A; 2. 6% BRCA 1/2 MUTYH CD 36 6. 5 9 4. 5 CHEK 2; 1. 0% FLCN; 1. 0% SDHA; 2. 4% MAP 3 K 7; 1. 0% TYK 2; 1. 0% CUX 1; 2. 1% TSC 2; 1. 9% VHL; 1. 2% PTCH 1; 1. 7% NOTCH 4; 1. 4% PDCD 11; 1. 6% ASXL 1; 1. 6% CCT 6 B; 1. 4% RET; 1. 4% TET 2; 1. 4% CONCLUSIONS GPS 1; 1. 4% TCF 3; 1. 4% • NGS can significantly impact the diagnosis and selection of targeted therapies in the treatment of sarcoma • NGS also facilitates the identification of the tumour mutational burden, which can aid in selecting immunotherapy • Further investigation is required regarding suspected germline aberrations Gounder MM, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11001

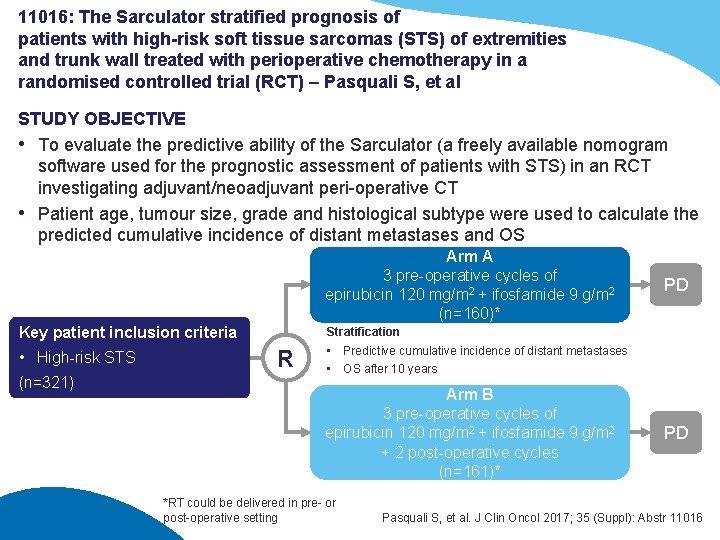
11016: The Sarculator stratified prognosis of patients with high-risk soft tissue sarcomas (STS) of extremities and trunk wall treated with perioperative chemotherapy in a randomised controlled trial (RCT) – Pasquali S, et al STUDY OBJECTIVE • To evaluate the predictive ability of the Sarculator (a freely available nomogram software used for the prognostic assessment of patients with STS) in an RCT investigating adjuvant/neoadjuvant peri-operative CT • Patient age, tumour size, grade and histological subtype were used to calculate the predicted cumulative incidence of distant metastases and OS Arm A 3 pre-operative cycles of epirubicin 120 mg/m 2 + ifosfamide 9 g/m 2 (n=160)* Key patient inclusion criteria • High-risk STS (n=321) PD Stratification R • Predictive cumulative incidence of distant metastases • OS after 10 years Arm B 3 pre-operative cycles of epirubicin 120 mg/m 2 + ifosfamide 9 g/m 2 + 2 post-operative cycles (n=161)* *RT could be delivered in pre- or post-operative setting PD Pasquali S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11016

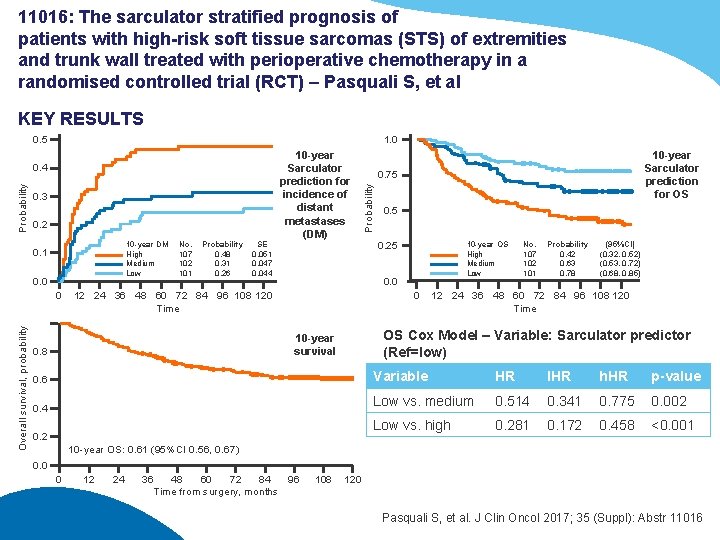
11016: The sarculator stratified prognosis of patients with high-risk soft tissue sarcomas (STS) of extremities and trunk wall treated with perioperative chemotherapy in a randomised controlled trial (RCT) – Pasquali S, et al KEY RESULTS 1. 0 0. 5 0. 3 0. 2 10 -year DM High Medium Low 0. 1 0. 0 Overall survival, probability 0 No. 107 102 101 Probability 0. 48 0. 31 0. 26 SE 0. 051 0. 047 0. 044 10 -year Sarculator prediction for incidence of distant metastases (DM) 0. 5 0. 25 10 -year OS High Medium Low 0. 0 12 24 36 48 60 72 84 96 108 120 Time 0 No. 107 102 101 Probability 0. 42 0. 63 0. 78 (95%CI) (0. 32, 0. 52) (0. 53, 0. 72) (0. 68, 0. 85) 12 24 36 48 60 72 84 96 108 120 Time OS Cox Model – Variable: Sarculator predictor (Ref=low) 10 -year survival 0. 8 10 -year Sarculator prediction for OS 0. 75 Probability 0. 4 0. 6 Variable HR IHR h. HR p-value 0. 4 Low vs. medium 0. 514 0. 341 0. 775 0. 002 Low vs. high 0. 281 0. 172 0. 458 <0. 001 0. 2 10 -year OS: 0. 61 (95%CI 0. 56, 0. 67) 0. 0 0 12 24 36 48 60 72 84 96 Time from surgery, months 108 120 Pasquali S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11016

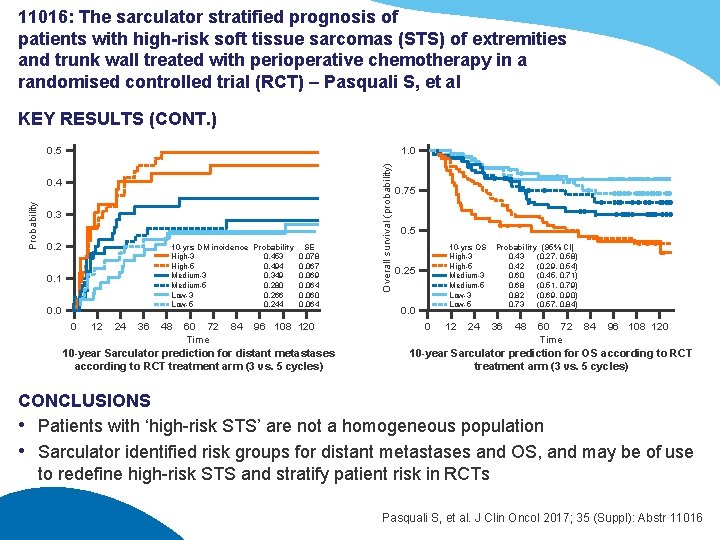
11016: The sarculator stratified prognosis of patients with high-risk soft tissue sarcomas (STS) of extremities and trunk wall treated with perioperative chemotherapy in a randomised controlled trial (RCT) – Pasquali S, et al KEY RESULTS (CONT. ) 0. 5 Probability 0. 4 0. 3 0. 2 10 -yrs DM incidence Probability SE High-3 0. 453 0. 078 High-5 0. 494 0. 067 Medium-3 0. 349 0. 069 Medium-5 0. 280 0. 064 Low-3 0. 266 0. 060 Low-5 0. 244 0. 064 0. 1 0. 0 0 12 24 36 48 60 72 84 96 108 120 Time 10 -year Sarculator prediction for distant metastases according to RCT treatment arm (3 vs. 5 cycles) Overall survival (probability) 1. 0 0. 75 0. 5 10 -yrs OS High-3 High-5 Medium-3 Medium-5 Low-3 Low-5 0. 25 0. 0 0 12 24 Probability (95% CI) 0. 43 (0. 27, 0. 58) 0. 42 (0. 29, 0. 54) 0. 60 (0. 45, 0. 71) 0. 68 (0. 51, 0. 79) 0. 82 (0. 69, 0. 90) 0. 73 (0. 57, 0. 84) 36 48 60 72 84 96 108 120 Time 10 -year Sarculator prediction for OS according to RCT treatment arm (3 vs. 5 cycles) CONCLUSIONS • Patients with ‘high-risk STS’ are not a homogeneous population • Sarculator identified risk groups for distant metastases and OS, and may be of use to redefine high-risk STS and stratify patient risk in RCTs Pasquali S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11016

11017: Factors impacting contemporary management of highgrade extremity sarcoma: An analysis of 12, 020 patients – Ramey S, et al STUDY OBJECTIVE • To investigate the differences (racial/ethnic) in local treatment received for highgrade extremity STS and their effect on OS, using data from a US National Cancer Database METHODS • Patients (n=12, 020) with stage II–III, high-grade extremity STS undergoing amputation (AMP) alone, limb-sparing surgery (LSS) alone, preoperative RT (pre. RT) + LSS or LSS + post-operative RT (post-RT) were selected from a National Cancer Database • Patterns of local treatment were identified through multivariate analyses using logistic regression • Cox proportional hazards regression was used to estimate OS, and the Kaplan. Meier method was used to estimate 5 -year OS Ramey S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11017

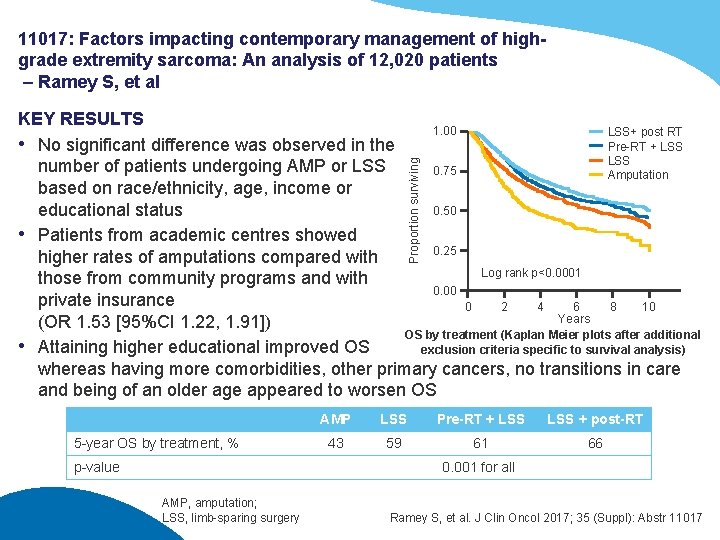
11017: Factors impacting contemporary management of highgrade extremity sarcoma: An analysis of 12, 020 patients – Ramey S, et al Proportion surviving KEY RESULTS 1. 00 LSS+ post RT Pre-RT + LSS • No significant difference was observed in the LSS number of patients undergoing AMP or LSS 0. 75 Amputation based on race/ethnicity, age, income or 0. 50 educational status • Patients from academic centres showed 0. 25 higher rates of amputations compared with Log rank p<0. 0001 those from community programs and with 0. 00 private insurance 0 2 4 6 8 10 Years (OR 1. 53 [95%CI 1. 22, 1. 91]) OS by treatment (Kaplan Meier plots after additional • Attaining higher educational improved OS exclusion criteria specific to survival analysis) whereas having more comorbidities, other primary cancers, no transitions in care and being of an older age appeared to worsen OS 5 -year OS by treatment, % p-value AMP LSS Pre-RT + LSS + post-RT 43 59 61 66 0. 001 for all AMP, amputation; LSS, limb-sparing surgery Ramey S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11017

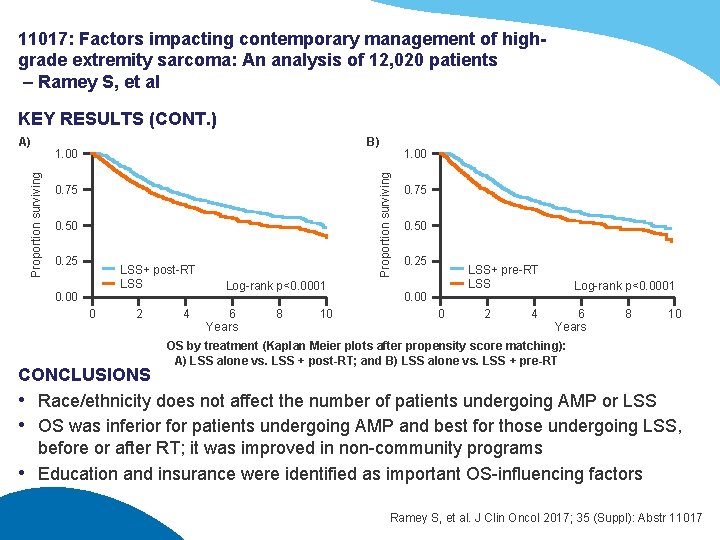
11017: Factors impacting contemporary management of highgrade extremity sarcoma: An analysis of 12, 020 patients – Ramey S, et al KEY RESULTS (CONT. ) B) 1. 00 0. 75 0. 50 0. 25 LSS+ post-RT LSS 0. 00 0 2 4 1. 00 Proportion surviving A) Log-rank p<0. 0001 6 Years 8 10 0. 75 0. 50 0. 25 LSS+ pre-RT LSS 0. 00 0 2 4 Log-rank p<0. 0001 6 Years 8 10 OS by treatment (Kaplan Meier plots after propensity score matching): A) LSS alone vs. LSS + post-RT; and B) LSS alone vs. LSS + pre-RT CONCLUSIONS • Race/ethnicity does not affect the number of patients undergoing AMP or LSS • OS was inferior for patients undergoing AMP and best for those undergoing LSS, before or after RT; it was improved in non-community programs • Education and insurance were identified as important OS-influencing factors Ramey S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11017

11019: Analysis of osteosarcoma subtypes by clinical genomic testing to identify clinically actionable alterations – Livingstone JA, et al STUDY OBJECTIVE • To assess the use of genomic testing to identify therapies for patients with recurrent/metastatic osteosarcoma and to assess actionable alterations across multiple histologic subtypes of osteosarcoma METHODS • Samples from 64 patients were subjected to standardized hotspot mutation analysis, using a 46 -, 50 - or 128 -gene CLIA certified multiplex platform • A 200 -gene analysis was performed for 18 patients using a companion research protocol • The Catalogue Of Somatic Mutations In Cancer (COSMIC) database was used to identify alterations in mutation hotspots in osteosarcoma and in other cancers and subsequently, clinical outcomes were investigated • Histologic subtype classifications were based on pre-treatment and resection specimens; cases with only metastatic samples were classed as ‘other high-grade osteosarcoma’ Livingstone JA, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11019

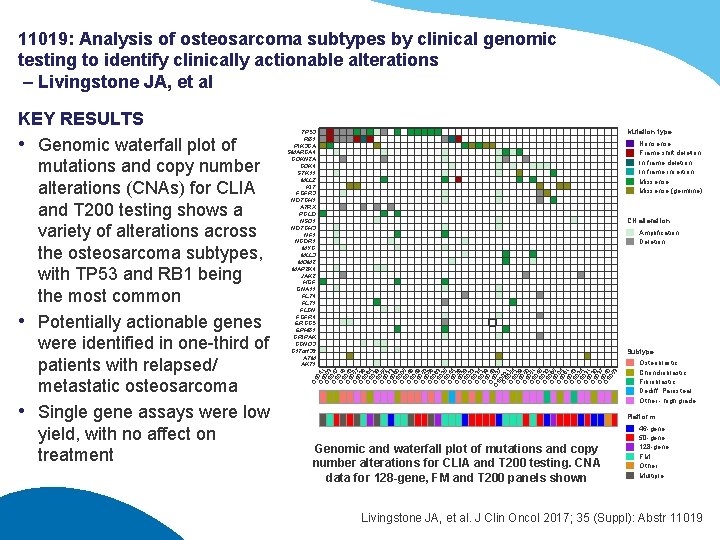
11019: Analysis of osteosarcoma subtypes by clinical genomic testing to identify clinically actionable alterations – Livingstone JA, et al Mutation type TP 53 RB 1 PIK 3 CA SMARCA 4 CDKN 2 A CDK 4 STK 11 MLL 2 KIT FGFR 3 NOTCH 1 ATRX PCLO NSD 1 NOTCH 3 NF 1 NCOR 1 MYC MLL 3 MDM 2 MAP 2 K 4 JAK 2 HGF GNA 11 FLT 4 FLT 1 FLCN FGFR 4 ERCC 5 EPHB 1 CRIPAK CCND 3 C 17 orf 39 ATM AKT 1 Nonsense Frame shift deletion In frame insertion Missense (germline) CN alteration Amplification Deletion Subtype OS OS 041 OS 003 OS 047 OS 016 OS 042 OS 057 OS 036 OS 064 OS 038 OS 055 OS 033 OS 060 OS 005 OS 046 OS 049 OS 052 OS 056 OS 063 OS 025 OS 044 OS 026 OS 028 OS 023 OS 034 OS 039 O 04 OSS 028 7 OS 002 OS 0514 OS 029 OS 050 OS 051 OS 045 OS 032 OS 065 OS 004 OS 061 OS 043 OS 024 OS 031 OS 030 OS 037 OS 040 05 3 KEY RESULTS • Genomic waterfall plot of mutations and copy number alterations (CNAs) for CLIA and T 200 testing shows a variety of alterations across the osteosarcoma subtypes, with TP 53 and RB 1 being the most common • Potentially actionable genes were identified in one-third of patients with relapsed/ metastatic osteosarcoma • Single gene assays were low yield, with no affect on treatment Osteoblastic Chondroblastic Fibroblastic Dediff. Parosteal Other - high grade Platform Genomic and waterfall plot of mutations and copy number alterations for CLIA and T 200 testing. CNA data for 128 -gene, FM and T 200 panels shown 46 -gene 50 -gene 128 -gene FM Other Multiple Livingstone JA, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11019

11019: Analysis of osteosarcoma subtypes by clinical genomic testing to identify clinically actionable alterations – Livingstone JA, et al KEY RESULTS (CONT. ) • Panel testing for CNAs had a high yield (potentially actionable 13/19, 68%) • Of 22 patients, tests results could be used to match subsequent therapies for only 2 patients – Matched therapy comprised m. TOR and PDGFR inhibition for a patient with NF 2 loss and CDK inhibition for a patient with CCNE 1 – Both patients showed disease progression as best response CONCLUSIONS • Due to the molecular heterogeneity of osteosarcoma and frequency of alterations identified during panel testing, clinical genomic testing may play an essential role in selecting patients for trials involving matched therapies • Future research is being conducted to identify hindrances for matched therapies when treating patients with osteosarcoma with potentially actionable alterations Livingstone JA, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11019

Locally advanced sarcoma

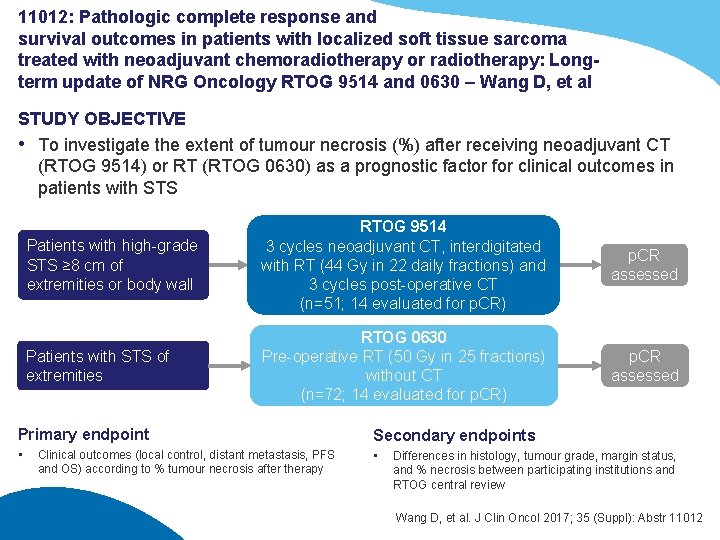
11012: Pathologic complete response and survival outcomes in patients with localized soft tissue sarcoma treated with neoadjuvant chemoradiotherapy or radiotherapy: Longterm update of NRG Oncology RTOG 9514 and 0630 – Wang D, et al STUDY OBJECTIVE • To investigate the extent of tumour necrosis (%) after receiving neoadjuvant CT (RTOG 9514) or RT (RTOG 0630) as a prognostic factor for clinical outcomes in patients with STS Patients with high-grade STS ≥ 8 cm of extremities or body wall RTOG 9514 3 cycles neoadjuvant CT, interdigitated with RT (44 Gy in 22 daily fractions) and 3 cycles post-operative CT (n=51; 14 evaluated for p. CR) p. CR assessed Patients with STS of extremities RTOG 0630 Pre-operative RT (50 Gy in 25 fractions) without CT (n=72; 14 evaluated for p. CR) p. CR assessed Primary endpoint Secondary endpoints • • Clinical outcomes (local control, distant metastasis, PFS and OS) according to % tumour necrosis after therapy Differences in histology, tumour grade, margin status, and % necrosis between participating institutions and RTOG central review Wang D, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11012

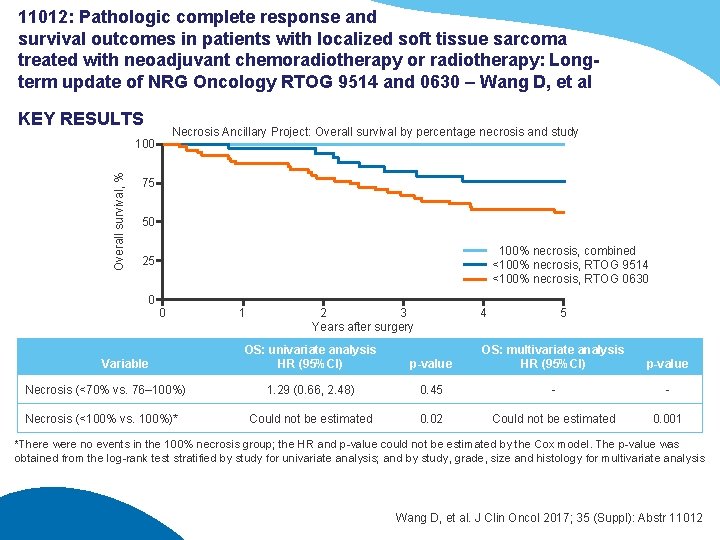
11012: Pathologic complete response and survival outcomes in patients with localized soft tissue sarcoma treated with neoadjuvant chemoradiotherapy or radiotherapy: Longterm update of NRG Oncology RTOG 9514 and 0630 – Wang D, et al KEY RESULTS Necrosis Ancillary Project: Overall survival by percentage necrosis and study Overall survival, % 100 75 50 100% necrosis, combined <100% necrosis, RTOG 9514 <100% necrosis, RTOG 0630 25 0 0 Variable Necrosis (<70% vs. 76– 100%) Necrosis (<100% vs. 100%)* 1 2 3 Years after surgery OS: univariate analysis HR (95%CI) 4 5 p-value OS: multivariate analysis HR (95%CI) p-value 1. 29 (0. 66, 2. 48) 0. 45 - - Could not be estimated 0. 02 Could not be estimated 0. 001 *There were no events in the 100% necrosis group; the HR and p-value could not be estimated by the Cox model. The p-value was obtained from the log-rank test stratified by study for univariate analysis; and by study, grade, size and histology for multivariate analysis Wang D, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11012

11012: Pathologic complete response and survival outcomes in patients with localized soft tissue sarcoma treated with neoadjuvant chemoradiotherapy or radiotherapy: Longterm update of NRG Oncology RTOG 9514 and 0630 – Wang D, et al KEY RESULTS (CONT. ) • Of 135 patients who had surgery, 123 were evaluable for p. CR with 14/51 (27. 5%) on RTOG 9514 and 14/72 (19. 4%) on 0630 having p. CR • Survival outcomes were superior in patients with p. CR • Local failure rate was 0% in patients with p. CR • In patients without p. CR, local failure at 5 years was 11. 7% and 9. 1% in RTOG 9514 and 0630, respectively • OS was improved in patients with leiomyosarcoma, myxoid/round cell liposarcoma and myxofibrosarcoma; liposarcoma/myxofibrosarcoma showed improved DFS with neoadjuvant CT CONCLUSIONS • p. CR improved survival outcomes in patients with STS treated with either neoadjuvant RT or CT-RT • In the future, p. CR may be considered as a survival surrogate in patients with STS if prospectively validated Wang D, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11012

11014: Clinical, radiological and genetic features, associated with the histopathologic response to neoadjuvant chemotherapy (NAC) and outcomes in locally advanced soft tissue sarcoma (STS) patients (pts) – Cousin S, et al STUDY OBJECTIVE • To evaluate the prognostic impact of histopathological response to NAC and the clinicopathological, radiological and genetic factors associated with this response METHODS • Patients (n=150) with localised STS of the trunk wall or extremities, confirmed by centralised histological review were included • Data on clinical and histological aspects, conventional and DCE-MRI at diagnosis and after 2 courses of NAC and genomic data from initial biopsy and post-NAC surgical specimens were collected • Good responders were defined as a post-NAC cellularity on surgery specimen <10% Primary endpoint • Factors associated with pathologic response Secondary endpoints • Factors associated with clinical outcomes (OS, PFS) Cousin S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11014

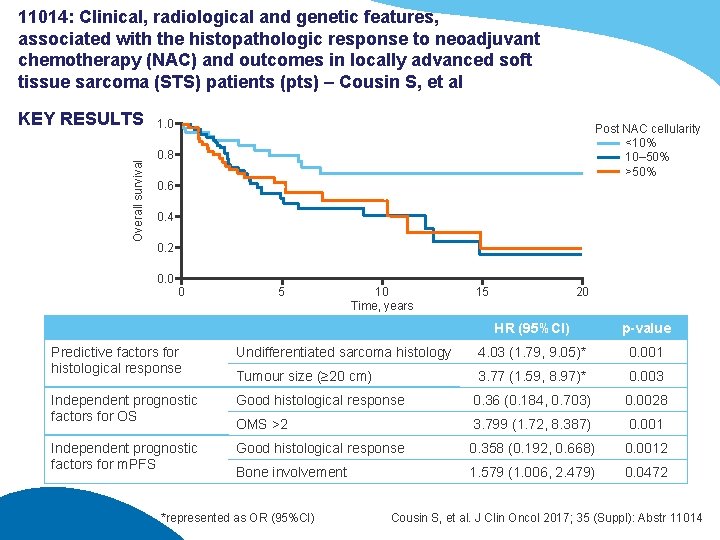
11014: Clinical, radiological and genetic features, associated with the histopathologic response to neoadjuvant chemotherapy (NAC) and outcomes in locally advanced soft tissue sarcoma (STS) patients (pts) – Cousin S, et al Overall survival KEY RESULTS 1. 0 Post NAC cellularity <10% 10– 50% >50% 0. 8 0. 6 0. 4 0. 2 0. 0 0 5 10 Time, years 15 20 HR (95%CI) p-value Predictive factors for histological response Undifferentiated sarcoma histology 4. 03 (1. 79, 9. 05)* 0. 001 Tumour size (≥ 20 cm) 3. 77 (1. 59, 8. 97)* 0. 003 Independent prognostic factors for OS Good histological response 0. 36 (0. 184, 0. 703) 0. 0028 OMS >2 3. 799 (1. 72, 8. 387) 0. 001 Independent prognostic factors for m. PFS Good histological response 0. 358 (0. 192, 0. 668) 0. 0012 Bone involvement 1. 579 (1. 006, 2. 479) 0. 0472 *represented as OR (95%CI) Cousin S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11014

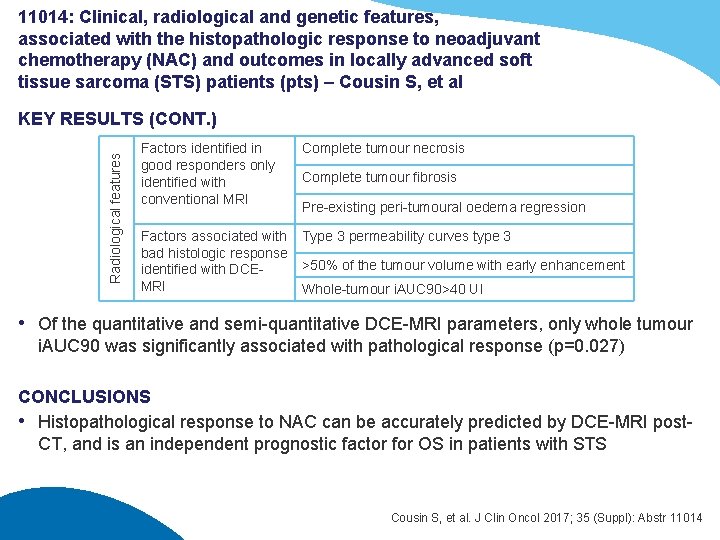
11014: Clinical, radiological and genetic features, associated with the histopathologic response to neoadjuvant chemotherapy (NAC) and outcomes in locally advanced soft tissue sarcoma (STS) patients (pts) – Cousin S, et al Radiological features KEY RESULTS (CONT. ) Factors identified in good responders only identified with conventional MRI Complete tumour necrosis Factors associated with bad histologic response identified with DCEMRI Type 3 permeability curves type 3 Complete tumour fibrosis Pre-existing peri-tumoural oedema regression >50% of the tumour volume with early enhancement Whole-tumour i. AUC 90>40 UI • Of the quantitative and semi-quantitative DCE-MRI parameters, only whole tumour i. AUC 90 was significantly associated with pathological response (p=0. 027) CONCLUSIONS • Histopathological response to NAC can be accurately predicted by DCE-MRI post. CT, and is an independent prognostic factor for OS in patients with STS Cousin S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11014

Advanced/metastatic sarcoma: chemotherapy, targeted therapy and immunotherapy

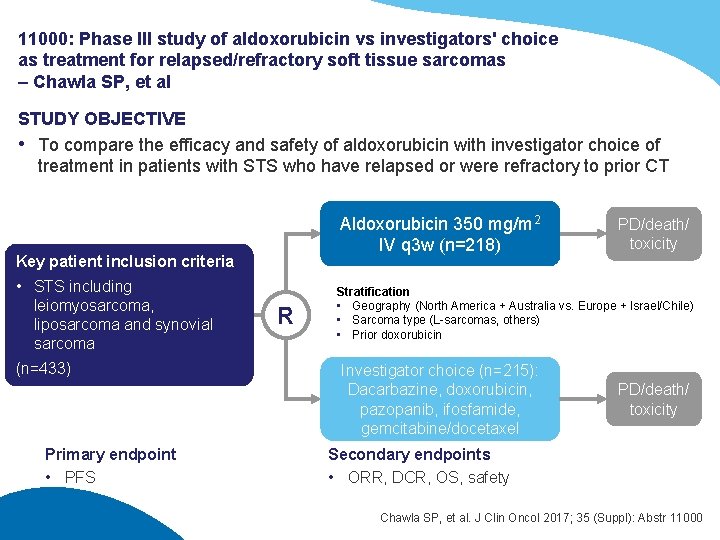
11000: Phase III study of aldoxorubicin vs investigators' choice as treatment for relapsed/refractory soft tissue sarcomas – Chawla SP, et al STUDY OBJECTIVE • To compare the efficacy and safety of aldoxorubicin with investigator choice of treatment in patients with STS who have relapsed or were refractory to prior CT Aldoxorubicin 350 mg/m 2 IV q 3 w (n=218) Key patient inclusion criteria • STS including leiomyosarcoma, liposarcoma and synovial sarcoma (n=433) Primary endpoint • PFS R PD/death/ toxicity Stratification • Geography (North America + Australia vs. Europe + Israel/Chile) • Sarcoma type (L-sarcomas, others) • Prior doxorubicin Investigator choice (n=215): Dacarbazine, doxorubicin, pazopanib, ifosfamide, gemcitabine/docetaxel PD/death/ toxicity Secondary endpoints • ORR, DCR, OS, safety Chawla SP, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11000

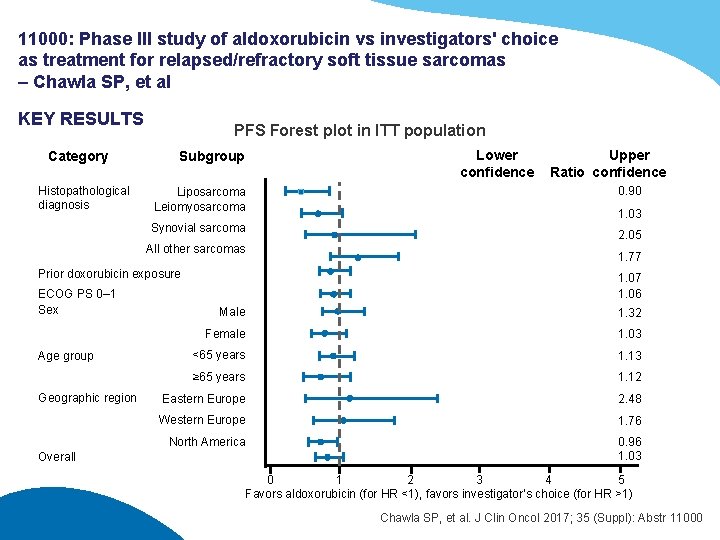
11000: Phase III study of aldoxorubicin vs investigators' choice as treatment for relapsed/refractory soft tissue sarcomas – Chawla SP, et al KEY RESULTS Category Histopathological diagnosis PFS Forest plot in ITT population Subgroup Liposarcoma Leiomyosarcoma Synovial sarcoma All other sarcomas Prior doxorubicin exposure ECOG PS 0– 1 Sex Age group Geographic region Overall Lower confidence Upper Ratio confidence 0. 90 1. 03 2. 05 1. 77 1. 06 Male 1. 32 Female 1. 03 <65 years 1. 13 ≥ 65 years 1. 12 Eastern Europe 2. 48 Western Europe 1. 76 North America 0. 96 1. 03 0 1 2 3 4 5 Favors aldoxorubicin (for HR <1), favors investigator’s choice (for HR >1) Chawla SP, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11000

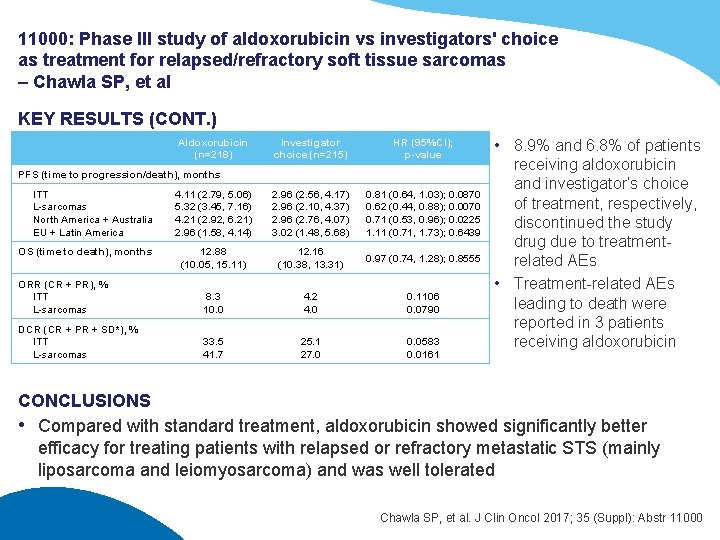
11000: Phase III study of aldoxorubicin vs investigators' choice as treatment for relapsed/refractory soft tissue sarcomas – Chawla SP, et al KEY RESULTS (CONT. ) Aldoxorubicin (n=218) Investigator choice (n=215) HR (95%CI); p-value 4. 11 (2. 79, 5. 06) 5. 32 (3. 45, 7. 16) 4. 21 (2. 92, 6. 21) 2. 96 (1. 58, 4. 14) 2. 96 (2. 56, 4. 17) 2. 96 (2. 10, 4. 37) 2. 96 (2. 76, 4. 07) 3. 02 (1. 48, 5. 68) 0. 81 (0. 64, 1. 03); 0. 0870 0. 62 (0. 44, 0. 88); 0. 0070 0. 71 (0. 53, 0. 96); 0. 0225 1. 11 (0. 71, 1. 73); 0. 6439 12. 88 (10. 05, 15. 11) 12. 16 (10. 38, 13. 31) 0. 97 (0. 74, 1. 28); 0. 8555 ORR (CR + PR), % ITT L-sarcomas 8. 3 10. 0 4. 2 4. 0 0. 1106 0. 0790 DCR (CR + PR + SD*), % ITT L-sarcomas 33. 5 41. 7 25. 1 27. 0 0. 0583 0. 0161 PFS (time to progression/death), months ITT L-sarcomas North America + Australia EU + Latin America OS (time to death), months • 8. 9% and 6. 8% of patients receiving aldoxorubicin and investigator’s choice of treatment, respectively, discontinued the study drug due to treatmentrelated AEs • Treatment-related AEs leading to death were reported in 3 patients receiving aldoxorubicin CONCLUSIONS • Compared with standard treatment, aldoxorubicin showed significantly better efficacy for treating patients with relapsed or refractory metastatic STS (mainly liposarcoma and leiomyosarcoma) and was well tolerated Chawla SP, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11000

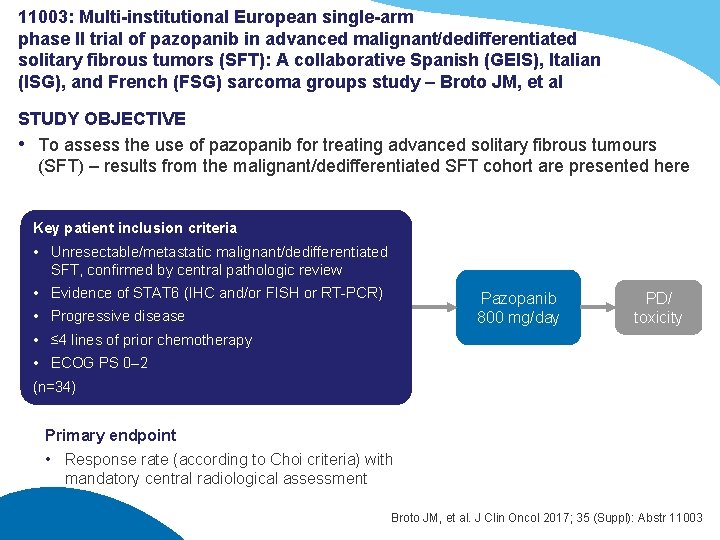
11003: Multi-institutional European single-arm phase II trial of pazopanib in advanced malignant/dedifferentiated solitary fibrous tumors (SFT): A collaborative Spanish (GEIS), Italian (ISG), and French (FSG) sarcoma groups study – Broto JM, et al STUDY OBJECTIVE • To assess the use of pazopanib for treating advanced solitary fibrous tumours (SFT) – results from the malignant/dedifferentiated SFT cohort are presented here Key patient inclusion criteria • Unresectable/metastatic malignant/dedifferentiated SFT, confirmed by central pathologic review • Evidence of STAT 6 (IHC and/or FISH or RT-PCR) Pazopanib 800 mg/day • Progressive disease PD/ toxicity • ≤ 4 lines of prior chemotherapy • ECOG PS 0– 2 (n=34) Primary endpoint • Response rate (according to Choi criteria) with mandatory central radiological assessment Broto JM, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11003

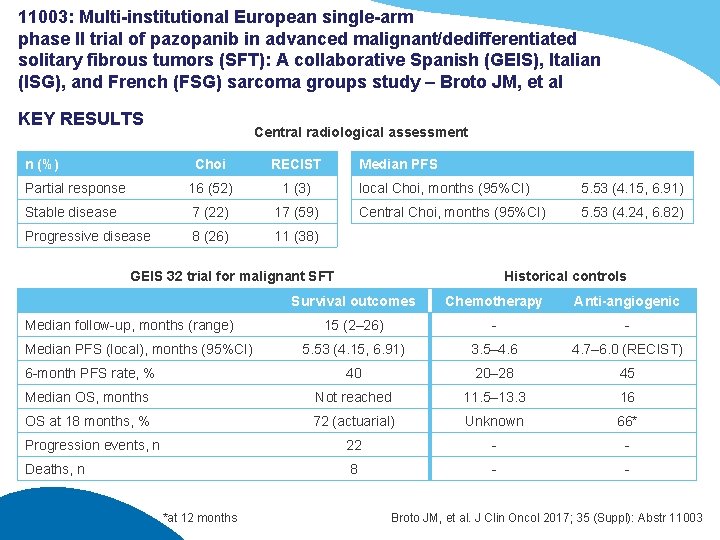
11003: Multi-institutional European single-arm phase II trial of pazopanib in advanced malignant/dedifferentiated solitary fibrous tumors (SFT): A collaborative Spanish (GEIS), Italian (ISG), and French (FSG) sarcoma groups study – Broto JM, et al KEY RESULTS n (%) Central radiological assessment Choi RECIST Partial response 16 (52) 1 (3) Stable disease 7 (22) 17 (59) Progressive disease 8 (26) 11 (38) Median PFS local Choi, months (95%CI) 5. 53 (4. 15, 6. 91) Central Choi, months (95%CI) 5. 53 (4. 24, 6. 82) GEIS 32 trial for malignant SFT Historical controls Survival outcomes Chemotherapy Anti-angiogenic 15 (2– 26) - - 5. 53 (4. 15, 6. 91) 3. 5– 4. 6 4. 7– 6. 0 (RECIST) 6 -month PFS rate, % 40 20– 28 45 Median OS, months Not reached 11. 5– 13. 3 16 OS at 18 months, % 72 (actuarial) Unknown 66* Progression events, n 22 - - Deaths, n 8 - - Median follow-up, months (range) Median PFS (local), months (95%CI) *at 12 months Broto JM, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11003

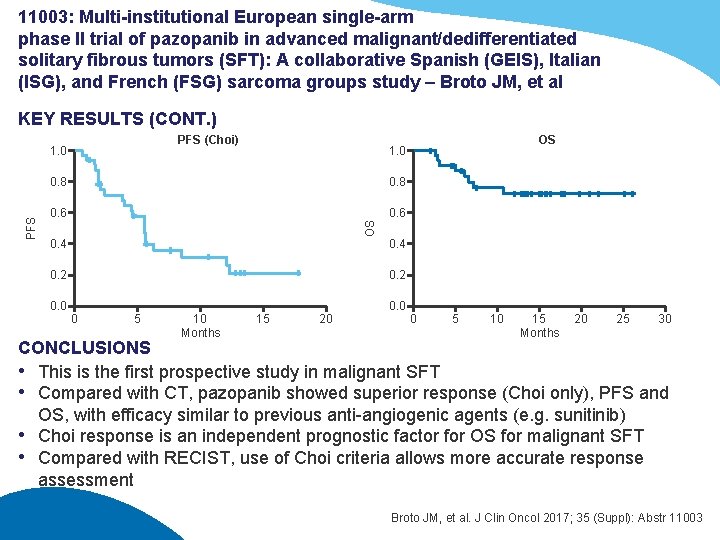
11003: Multi-institutional European single-arm phase II trial of pazopanib in advanced malignant/dedifferentiated solitary fibrous tumors (SFT): A collaborative Spanish (GEIS), Italian (ISG), and French (FSG) sarcoma groups study – Broto JM, et al KEY RESULTS (CONT. ) PFS (Choi) OS 1. 0 0. 8 0. 6 OS PFS 1. 0 0. 4 0. 2 0. 0 0 5 10 Months 15 20 0. 0 0 5 10 15 Months 20 25 30 CONCLUSIONS • This is the first prospective study in malignant SFT • Compared with CT, pazopanib showed superior response (Choi only), PFS and OS, with efficacy similar to previous anti-angiogenic agents (e. g. sunitinib) • Choi response is an independent prognostic factor for OS for malignant SFT • Compared with RECIST, use of Choi criteria allows more accurate response assessment Broto JM, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11003

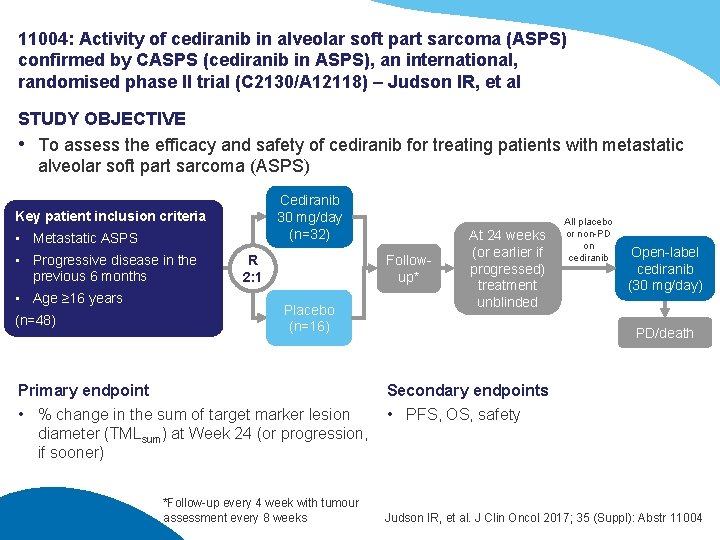
11004: Activity of cediranib in alveolar soft part sarcoma (ASPS) confirmed by CASPS (cediranib in ASPS), an international, randomised phase II trial (C 2130/A 12118) – Judson IR, et al STUDY OBJECTIVE • To assess the efficacy and safety of cediranib for treating patients with metastatic alveolar soft part sarcoma (ASPS) Cediranib 30 mg/day (n=32) Key patient inclusion criteria • Metastatic ASPS • Progressive disease in the previous 6 months • Age ≥ 16 years (n=48) R 2: 1 Followup* Placebo (n=16) Primary endpoint • % change in the sum of target marker lesion diameter (TMLsum) at Week 24 (or progression, if sooner) *Follow-up every 4 week with tumour assessment every 8 weeks At 24 weeks (or earlier if progressed) treatment unblinded All placebo or non-PD on cediranib Open-label cediranib (30 mg/day) PD/death Secondary endpoints • PFS, OS, safety Judson IR, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11004

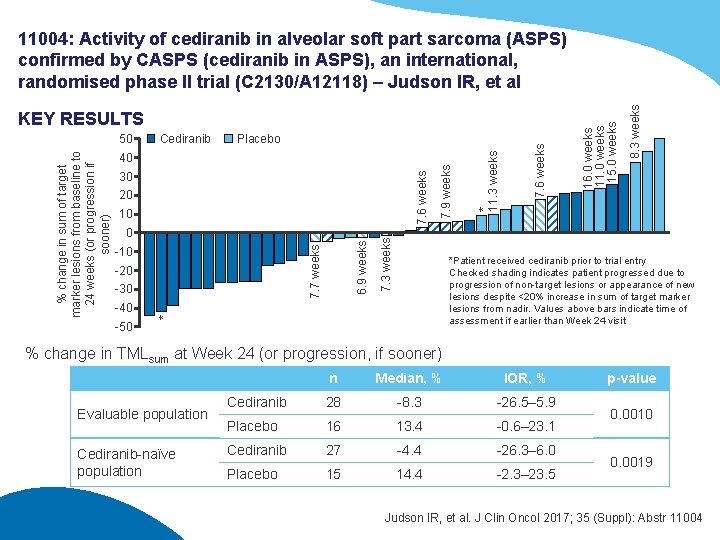
7. 6 weeks 30 20 7. 7 weeks 6. 9 weeks 0 -10 -20 -30 7. 3 weeks 10 7. 9 weeks 40 8. 3 weeks Placebo * 11. 3 weeks Cediranib *Patient received cediranib prior to trial entry Checked shading indicates patient progressed due to progression of non-target lesions or appearance of new lesions despite <20% increase in sum of target marker lesions from nadir. Values above bars indicate time of assessment if earlier than Week 24 visit -40 * % change in sum of target marker lesions from baseline to 24 weeks (or progression if sooner) 50 7. 6 weeks KEY RESULTS 16. 0 weeks 11. 0 weeks 15. 0 weeks 11004: Activity of cediranib in alveolar soft part sarcoma (ASPS) confirmed by CASPS (cediranib in ASPS), an international, randomised phase II trial (C 2130/A 12118) – Judson IR, et al -50 % change in TMLsum at Week 24 (or progression, if sooner) Evaluable population Cediranib-naïve population n Median, % IQR, % Cediranib 28 -8. 3 -26. 5– 5. 9 Placebo 16 13. 4 -0. 6– 23. 1 Cediranib 27 -4. 4 -26. 3– 6. 0 Placebo 15 14. 4 -2. 3– 23. 5 p-value 0. 0010 0. 0019 Judson IR, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11004

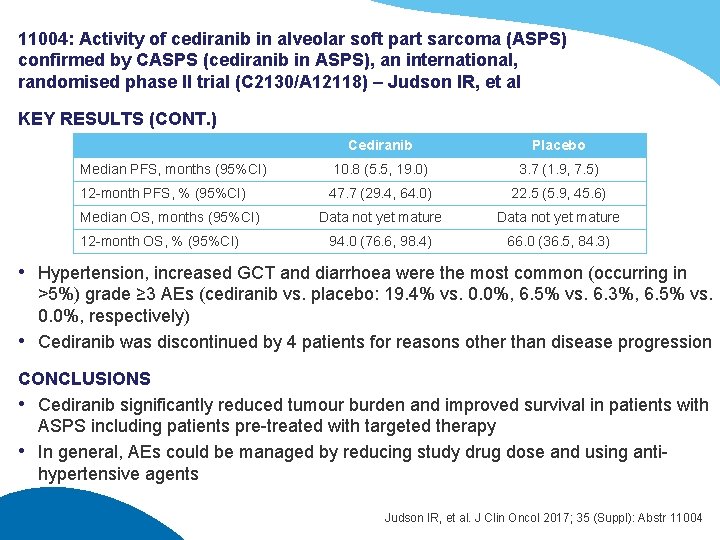
11004: Activity of cediranib in alveolar soft part sarcoma (ASPS) confirmed by CASPS (cediranib in ASPS), an international, randomised phase II trial (C 2130/A 12118) – Judson IR, et al KEY RESULTS (CONT. ) Cediranib Placebo Median PFS, months (95%CI) 10. 8 (5. 5, 19. 0) 3. 7 (1. 9, 7. 5) 12 -month PFS, % (95%CI) 47. 7 (29. 4, 64. 0) 22. 5 (5. 9, 45. 6) Data not yet mature 94. 0 (76. 6, 98. 4) 66. 0 (36. 5, 84. 3) Median OS, months (95%CI) 12 -month OS, % (95%CI) • Hypertension, increased GCT and diarrhoea were the most common (occurring in >5%) grade ≥ 3 AEs (cediranib vs. placebo: 19. 4% vs. 0. 0%, 6. 5% vs. 6. 3%, 6. 5% vs. 0. 0%, respectively) • Cediranib was discontinued by 4 patients for reasons other than disease progression CONCLUSIONS • Cediranib significantly reduced tumour burden and improved survival in patients with ASPS including patients pre-treated with targeted therapy • In general, AEs could be managed by reducing study drug dose and using antihypertensive agents Judson IR, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11004

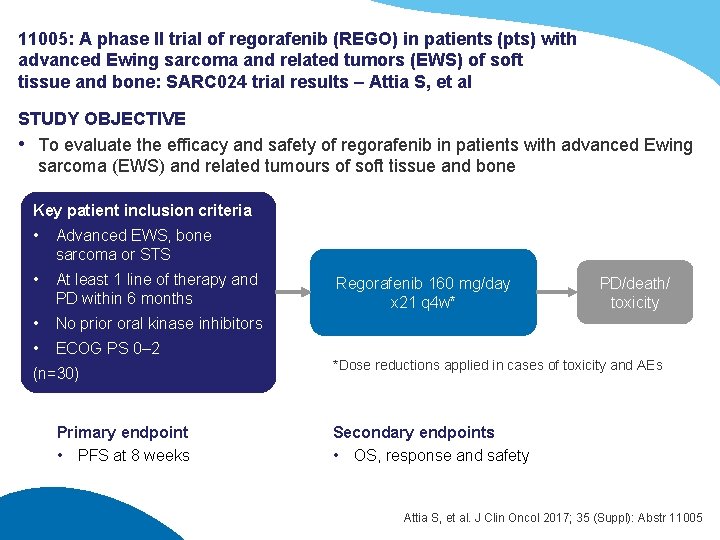
11005: A phase II trial of regorafenib (REGO) in patients (pts) with advanced Ewing sarcoma and related tumors (EWS) of soft tissue and bone: SARC 024 trial results – Attia S, et al STUDY OBJECTIVE • To evaluate the efficacy and safety of regorafenib in patients with advanced Ewing sarcoma (EWS) and related tumours of soft tissue and bone Key patient inclusion criteria • Advanced EWS, bone sarcoma or STS • At least 1 line of therapy and PD within 6 months • No prior oral kinase inhibitors • ECOG PS 0– 2 (n=30) Primary endpoint • PFS at 8 weeks Regorafenib 160 mg/day x 21 q 4 w* PD/death/ toxicity *Dose reductions applied in cases of toxicity and AEs Secondary endpoints • OS, response and safety Attia S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11005

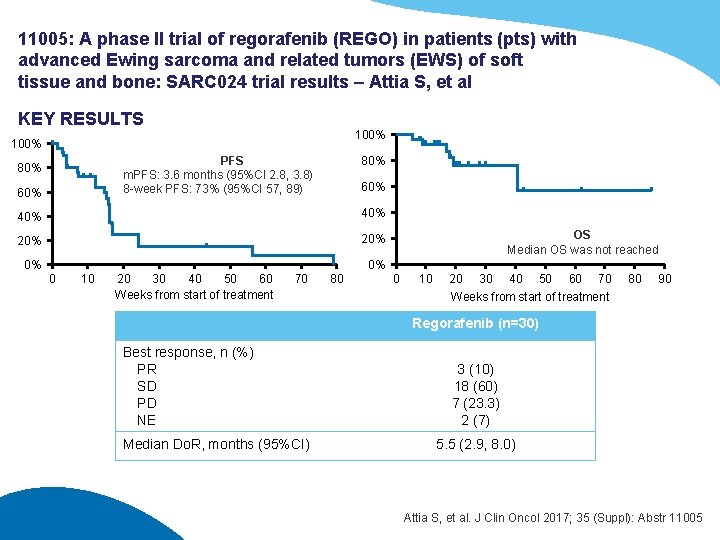
11005: A phase II trial of regorafenib (REGO) in patients (pts) with advanced Ewing sarcoma and related tumors (EWS) of soft tissue and bone: SARC 024 trial results – Attia S, et al KEY RESULTS 100% PFS m. PFS: 3. 6 months (95%CI 2. 8, 3. 8) 8 -week PFS: 73% (95%CI 57, 89) 80% 60% 40% 20% 0% OS Median OS was not reached 0% 0 10 20 30 40 50 60 Weeks from start of treatment 70 80 0 10 20 30 40 50 60 70 Weeks from start of treatment 80 90 Regorafenib (n=30) Best response, n (%) PR SD PD NE Median Do. R, months (95%CI) 3 (10) 18 (60) 7 (23. 3) 2 (7) 5. 5 (2. 9, 8. 0) Attia S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11005

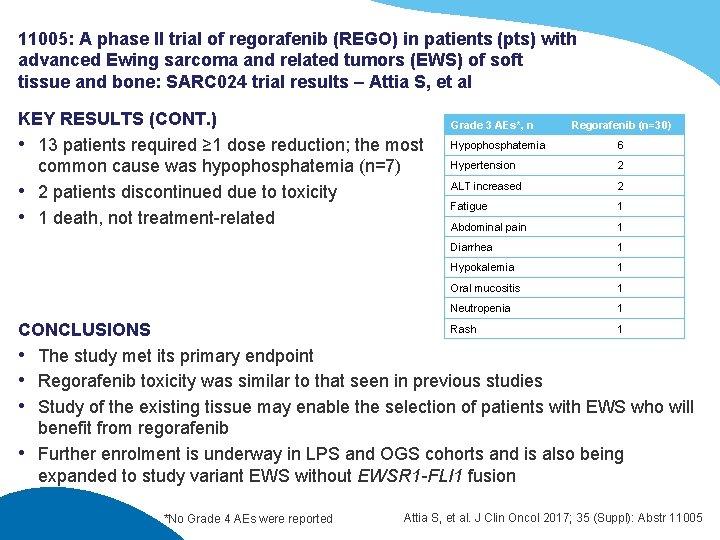
11005: A phase II trial of regorafenib (REGO) in patients (pts) with advanced Ewing sarcoma and related tumors (EWS) of soft tissue and bone: SARC 024 trial results – Attia S, et al KEY RESULTS (CONT. ) • 13 patients required ≥ 1 dose reduction; the most common cause was hypophosphatemia (n=7) • 2 patients discontinued due to toxicity • 1 death, not treatment-related Grade 3 AEs*, n Regorafenib (n=30) Hypophosphatemia 6 Hypertension 2 ALT increased 2 Fatigue 1 Abdominal pain 1 Diarrhea 1 Hypokalemia 1 Oral mucositis 1 Neutropenia 1 Rash 1 CONCLUSIONS • The study met its primary endpoint • Regorafenib toxicity was similar to that seen in previous studies • Study of the existing tissue may enable the selection of patients with EWS who will benefit from regorafenib • Further enrolment is underway in LPS and OGS cohorts and is also being expanded to study variant EWS without EWSR 1 -FLI 1 fusion *No Grade 4 AEs were reported Attia S, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11005

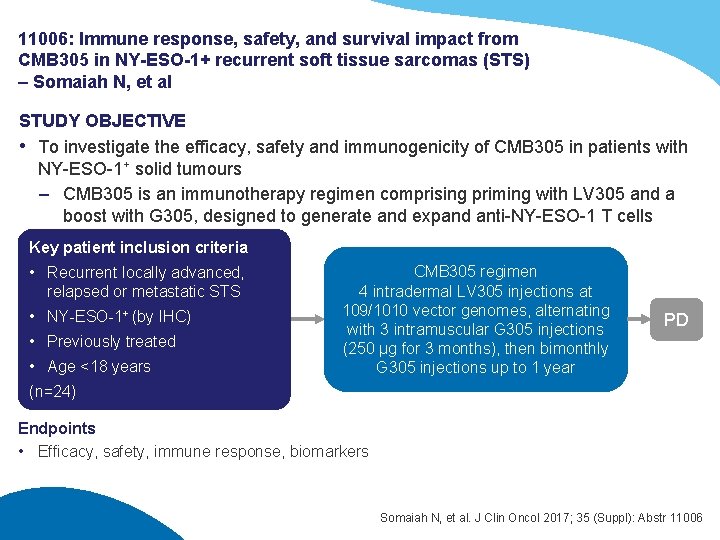
11006: Immune response, safety, and survival impact from CMB 305 in NY-ESO-1+ recurrent soft tissue sarcomas (STS) – Somaiah N, et al STUDY OBJECTIVE • To investigate the efficacy, safety and immunogenicity of CMB 305 in patients with NY-ESO-1+ solid tumours – CMB 305 is an immunotherapy regimen comprising priming with LV 305 and a boost with G 305, designed to generate and expand anti-NY-ESO-1 T cells Key patient inclusion criteria • Recurrent locally advanced, relapsed or metastatic STS • NY-ESO-1+ (by IHC) • Previously treated • Age <18 years CMB 305 regimen 4 intradermal LV 305 injections at 109/1010 vector genomes, alternating with 3 intramuscular G 305 injections (250 µg for 3 months), then bimonthly G 305 injections up to 1 year PD (n=24) Endpoints • Efficacy, safety, immune response, biomarkers Somaiah N, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11006

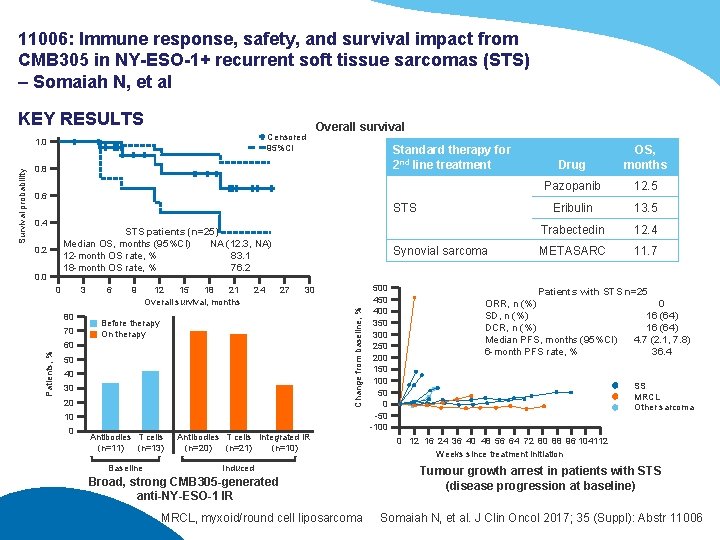
11006: Immune response, safety, and survival impact from CMB 305 in NY-ESO-1+ recurrent soft tissue sarcomas (STS) – Somaiah N, et al KEY RESULTS Censored 95%CI Standard therapy for 2 nd line treatment 0. 8 0. 6 STS 0. 4 STS patients (n=25) Median OS, months (95%CI) NA (12. 3, NA) 12 -month OS rate, % 83. 1 18 -month OS rate, % 76. 2 0. 0 0 3 80 70 6 9 12 15 18 21 Overall survival, months 24 Synovial sarcoma 27 30 Change from baseline, % 0. 2 Before therapy On therapy 60 Patients, % Survival probability 1. 0 Overall survival 50 40 30 20 10 0 Antibodies T cells (n=11) (n=13) Baseline Antibodies T cells Integrated IR (n=20) (n=21) (n=10) Induced Broad, strong CMB 305 -generated anti-NY-ESO-1 IR MRCL, myxoid/round cell liposarcoma 500 450 400 350 300 250 200 150 100 50 0 -50 -100 Drug OS, months Pazopanib 12. 5 Eribulin 13. 5 Trabectedin 12. 4 METASARC 11. 7 Patients with STS n=25 ORR, n (%) 0 SD, n (%) 16 (64) DCR, n (%) 16 (64) Median PFS, months (95%CI) 4. 7 (2. 1, 7. 8) 6 -month PFS rate, % 36. 4 SS MRCL Other sarcoma 0 12 16 24 36 40 48 56 64 72 80 88 96 104112 Weeks since treatment initiation Tumour growth arrest in patients with STS (disease progression at baseline) Somaiah N, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11006

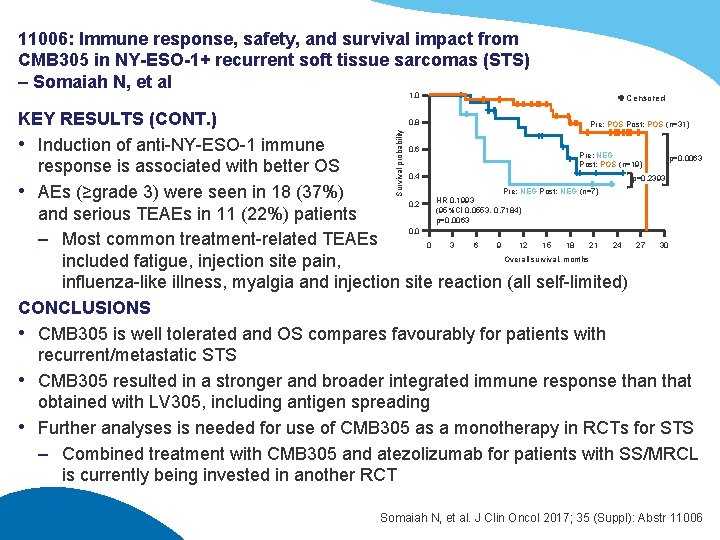
11006: Immune response, safety, and survival impact from CMB 305 in NY-ESO-1+ recurrent soft tissue sarcomas (STS) – Somaiah N, et al 1. 0 Censored Survival probability KEY RESULTS (CONT. ) 0. 8 Pre: POS Post: POS (n=31) • Induction of anti-NY-ESO-1 immune 0. 6 Pre: NEG p=0. 0063 Post: POS (n=19) response is associated with better OS 0. 4 p=0. 2393 Pre: NEG Post: NEG (n=7) • AEs (≥grade 3) were seen in 18 (37%) HR 0. 1993 0. 2 (95%CI 0. 0553, 0. 7184) and serious TEAEs in 11 (22%) patients p=0. 0063 0. 0 – Most common treatment-related TEAEs 0 3 6 9 12 15 18 21 24 27 30 Overall survival, months included fatigue, injection site pain, influenza-like illness, myalgia and injection site reaction (all self-limited) CONCLUSIONS • CMB 305 is well tolerated and OS compares favourably for patients with recurrent/metastatic STS • CMB 305 resulted in a stronger and broader integrated immune response than that obtained with LV 305, including antigen spreading • Further analyses is needed for use of CMB 305 as a monotherapy in RCTs for STS – Combined treatment with CMB 305 and atezolizumab for patients with SS/MRCL is currently being invested in another RCT Somaiah N, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11006

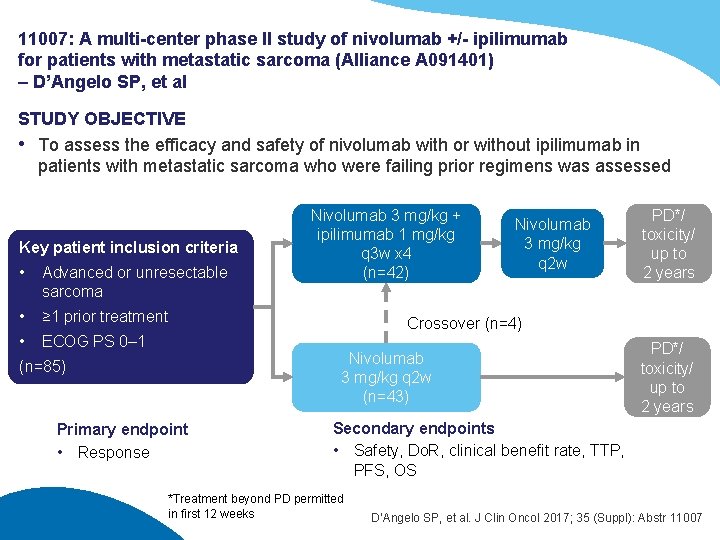
11007: A multi-center phase II study of nivolumab +/- ipilimumab for patients with metastatic sarcoma (Alliance A 091401) – D’Angelo SP, et al STUDY OBJECTIVE • To assess the efficacy and safety of nivolumab with or without ipilimumab in patients with metastatic sarcoma who were failing prior regimens was assessed Key patient inclusion criteria • Advanced or unresectable sarcoma • ≥ 1 prior treatment • ECOG PS 0– 1 Nivolumab 3 mg/kg + ipilimumab 1 mg/kg q 3 w x 4 (n=42) Nivolumab 3 mg/kg q 2 w PD*/ toxicity/ up to 2 years Crossover (n=4) Nivolumab 3 mg/kg q 2 w (n=43) (n=85) Primary endpoint • Response PD*/ toxicity/ up to 2 years Secondary endpoints • Safety, Do. R, clinical benefit rate, TTP, PFS, OS *Treatment beyond PD permitted in first 12 weeks D’Angelo SP, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11007

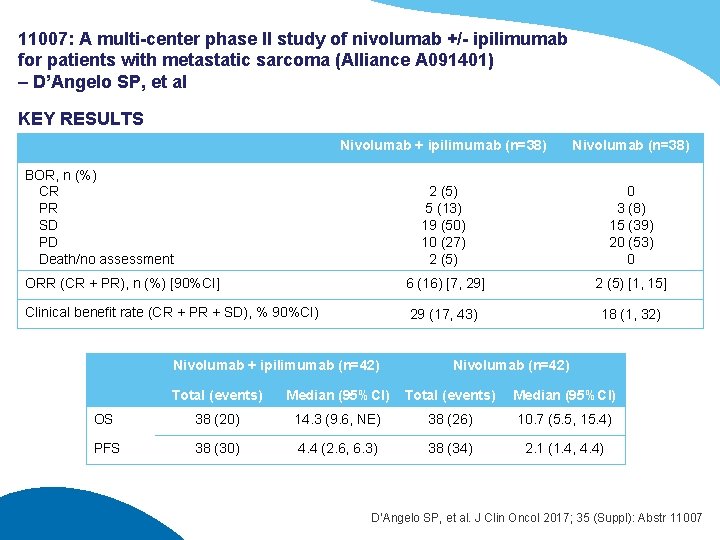
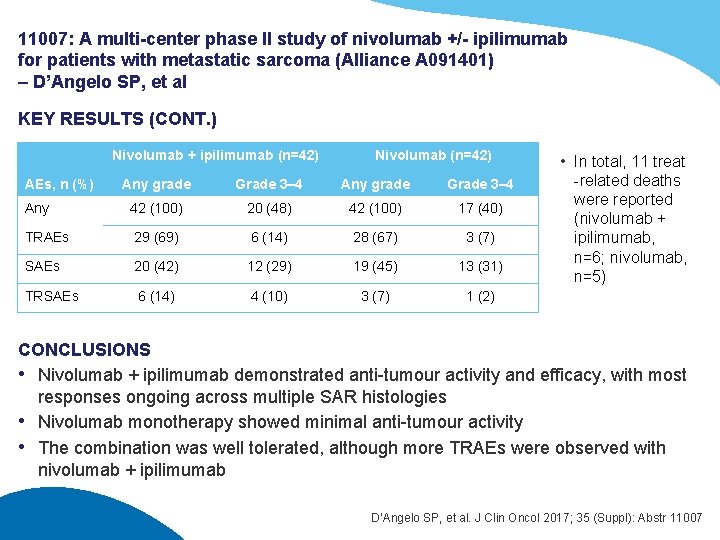
11007: A multi-center phase II study of nivolumab +/- ipilimumab for patients with metastatic sarcoma (Alliance A 091401) – D’Angelo SP, et al KEY RESULTS Nivolumab + ipilimumab (n=38) Nivolumab (n=38) 2 (5) 5 (13) 19 (50) 10 (27) 2 (5) 0 3 (8) 15 (39) 20 (53) 0 ORR (CR + PR), n (%) [90%CI] 6 (16) [7, 29] 2 (5) [1, 15] Clinical benefit rate (CR + PR + SD), % 90%CI) 29 (17, 43) 18 (1, 32) BOR, n (%) CR PR SD PD Death/no assessment Nivolumab + ipilimumab (n=42) Nivolumab (n=42) Total (events) Median (95%CI) OS 38 (20) 14. 3 (9. 6, NE) 38 (26) 10. 7 (5. 5, 15. 4) PFS 38 (30) 4. 4 (2. 6, 6. 3) 38 (34) 2. 1 (1. 4, 4. 4) D’Angelo SP, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11007

11007: A multi-center phase II study of nivolumab +/- ipilimumab for patients with metastatic sarcoma (Alliance A 091401) – D’Angelo SP, et al KEY RESULTS (CONT. ) Nivolumab + ipilimumab (n=42) Nivolumab (n=42) Any grade Grade 3– 4 Any 42 (100) 20 (48) 42 (100) 17 (40) TRAEs 29 (69) 6 (14) 28 (67) 3 (7) SAEs 20 (42) 12 (29) 19 (45) 13 (31) TRSAEs 6 (14) 4 (10) 3 (7) 1 (2) AEs, n (%) • In total, 11 treat -related deaths were reported (nivolumab + ipilimumab, n=6; nivolumab, n=5) CONCLUSIONS • Nivolumab + ipilimumab demonstrated anti-tumour activity and efficacy, with most responses ongoing across multiple SAR histologies • Nivolumab monotherapy showed minimal anti-tumour activity • The combination was well tolerated, although more TRAEs were observed with nivolumab + ipilimumab D’Angelo SP, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11007

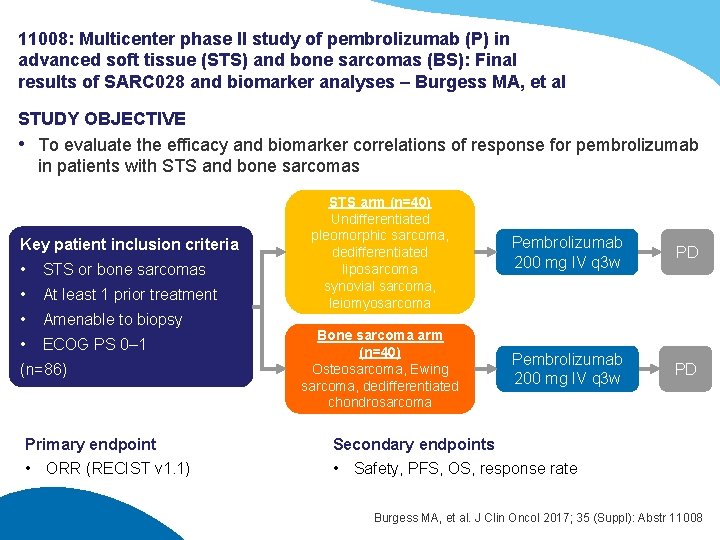
11008: Multicenter phase II study of pembrolizumab (P) in advanced soft tissue (STS) and bone sarcomas (BS): Final results of SARC 028 and biomarker analyses – Burgess MA, et al STUDY OBJECTIVE • To evaluate the efficacy and biomarker correlations of response for pembrolizumab in patients with STS and bone sarcomas Key patient inclusion criteria • STS or bone sarcomas • At least 1 prior treatment • Amenable to biopsy • ECOG PS 0– 1 (n=86) Primary endpoint • ORR (RECIST v 1. 1) STS arm (n=40) Undifferentiated pleomorphic sarcoma, dedifferentiated liposarcoma synovial sarcoma, leiomyosarcoma Pembrolizumab 200 mg IV q 3 w PD Bone sarcoma arm (n=40) Osteosarcoma, Ewing sarcoma, dedifferentiated chondrosarcoma Pembrolizumab 200 mg IV q 3 w PD Secondary endpoints • Safety, PFS, OS, response rate Burgess MA, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11008

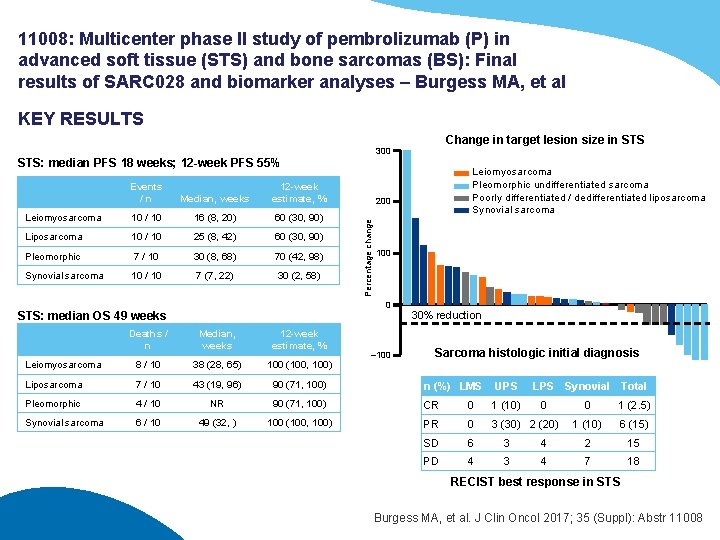
11008: Multicenter phase II study of pembrolizumab (P) in advanced soft tissue (STS) and bone sarcomas (BS): Final results of SARC 028 and biomarker analyses – Burgess MA, et al KEY RESULTS Change in target lesion size in STS 300 STS: median PFS 18 weeks; 12 -week PFS 55% Median, weeks 12 -week estimate, % Leiomyosarcoma 10 / 10 16 (8, 20) 60 (30, 90) Liposarcoma 10 / 10 25 (8, 42) 60 (30, 90) Pleomorphic 7 / 10 30 (8, 68) 70 (42, 98) Synovial sarcoma 10 / 10 7 (7, 22) 30 (2, 58) 200 Percentage change Events /n 100 0 STS: median OS 49 weeks Leiomyosarcoma Pleomorphic undifferentiated sarcoma Poorly differentiated / dedifferentiated liposarcoma Synovial sarcoma 30% reduction Deaths / n Median, weeks 12 -week estimate, % Leiomyosarcoma 8 / 10 38 (28, 65) 100 (100, 100) Liposarcoma 7 / 10 43 (19, 96) 90 (71, 100) n (%) LMS UPS Pleomorphic 4 / 10 NR 90 (71, 100) CR 0 1 (10) Synovial sarcoma 6 / 10 49 (32, ) 100 (100, 100) PR 0 3 (30) 2 (20) SD 6 3 PD 4 3 – 100 Sarcoma histologic initial diagnosis LPS Synovial Total 0 0 1 (2. 5) 1 (10) 6 (15) 4 2 15 4 7 18 RECIST best response in STS Burgess MA, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11008

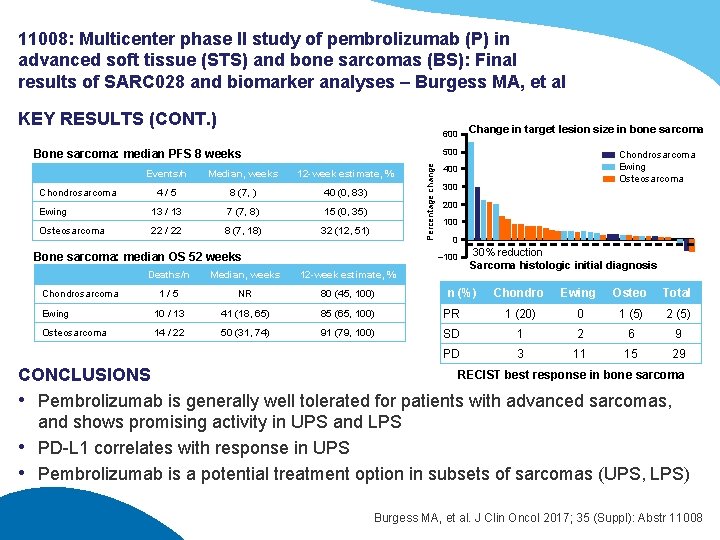
11008: Multicenter phase II study of pembrolizumab (P) in advanced soft tissue (STS) and bone sarcomas (BS): Final results of SARC 028 and biomarker analyses – Burgess MA, et al KEY RESULTS (CONT. ) 600 500 Events/n Median, weeks 12 -week estimate, % 4/5 8 (7, ) 40 (0, 83) Ewing 13 / 13 7 (7, 8) 15 (0, 35) Osteosarcoma 22 / 22 8 (7, 18) 32 (12, 51) Bone sarcoma: median OS 52 weeks Median, weeks 12 -week estimate, % 1/5 NR 80 (45, 100) Ewing 10 / 13 41 (18, 65) 85 (65, 100) Osteosarcoma 14 / 22 50 (31, 74) 91 (79, 100) Chondrosarcoma Ewing Osteosarcoma 400 300 200 100 0 – 100 Deaths/n Chondrosarcoma Percentage change Bone sarcoma: median PFS 8 weeks Chondrosarcoma Change in target lesion size in bone sarcoma 30% reduction Sarcoma histologic initial diagnosis n (%) Chondro Ewing Osteo Total PR 1 (20) 0 1 (5) 2 (5) SD 1 2 6 9 PD 3 11 15 29 RECIST best response in bone sarcoma CONCLUSIONS • Pembrolizumab is generally well tolerated for patients with advanced sarcomas, and shows promising activity in UPS and LPS • PD-L 1 correlates with response in UPS • Pembrolizumab is a potential treatment option in subsets of sarcomas (UPS, LPS) Burgess MA, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 11008

3000: Open label, non-randomized, multi-cohort pilot study of genetically engineered NY-ESO-1 specific NY-ESO 1 c 259 T in HLA-A 2+ patients with synovial sarcoma (NCT 01343043) – Mackall C, et al STUDY OBJECTIVE • To evaluate the safety and efficacy of NY-ESO-1 c 259 T cells recognizing an NY-ESO 1 -derived peptide complexed with HLA-A*02 in patients with synovial sarcoma METHODS • Eligible patients were positive for HLA-A*02: 01, 02: 05 or 02: 06, and had unresectable, metastatic or recurrent synovial sarcoma expressing NY-ESO-1 • Lymphocytes were obtained via leukapheresis, isolated, activated, transduced to express NY-ESO-1 c 259 T cell receptors and expanded. After lymphodepletion, T cells were infused at a target dose of 1– 6 × 109 cells • Disease was assessed at Weeks 4, 8 and 12 post-T-cell infusion, and subsequently at every 3 months Primary endpoint • ORR (CR + PR), evaluated in high* and low† NY-ESO-1 expressers with different lymphodepleting regimens *≥ 50% tumour cells express 2+/3+; †≥ 1+ in ≥ 1% cells, not exceeding 2+/3+ in ≥ 50% cells Secondary endpoints • Safety, Do. R, PFS, OS, gene-marked cell persistence Mackall C, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 3000

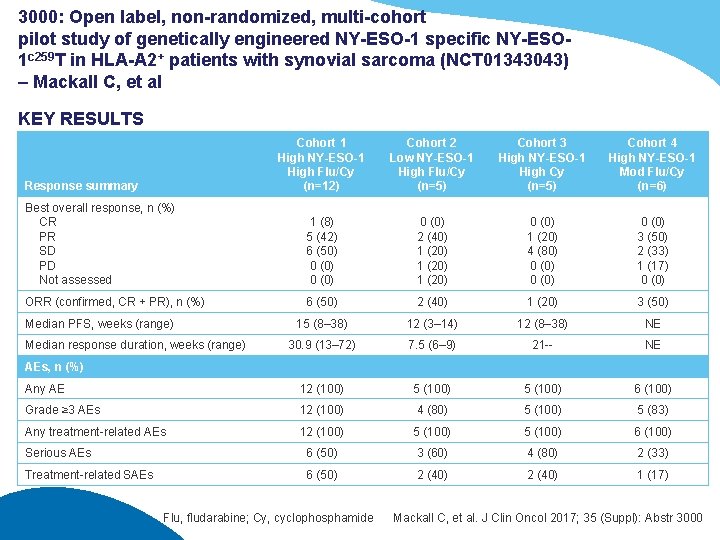
3000: Open label, non-randomized, multi-cohort pilot study of genetically engineered NY-ESO-1 specific NY-ESO 1 c 259 T in HLA-A 2+ patients with synovial sarcoma (NCT 01343043) – Mackall C, et al KEY RESULTS Cohort 1 High NY-ESO-1 High Flu/Cy (n=12) Cohort 2 Low NY-ESO-1 High Flu/Cy (n=5) Cohort 3 High NY-ESO-1 High Cy (n=5) Cohort 4 High NY-ESO-1 Mod Flu/Cy (n=6) Best overall response, n (%) CR PR SD PD Not assessed 1 (8) 5 (42) 6 (50) 0 (0) 2 (40) 1 (20) 0 (0) 1 (20) 4 (80) 0 (0) 3 (50) 2 (33) 1 (17) 0 (0) ORR (confirmed, CR + PR), n (%) 6 (50) 2 (40) 1 (20) 3 (50) 15 (8– 38) 12 (3– 14) 12 (8– 38) NE 30. 9 (13– 72) 7. 5 (6– 9) 21 -- NE Any AE 12 (100) 5 (100) 6 (100) Grade ≥ 3 AEs 12 (100) 4 (80) 5 (100) 5 (83) Any treatment-related AEs 12 (100) 5 (100) 6 (100) Serious AEs 6 (50) 3 (60) 4 (80) 2 (33) Treatment-related SAEs 6 (50) 2 (40) 1 (17) Response summary Median PFS, weeks (range) Median response duration, weeks (range) AEs, n (%) Flu, fludarabine; Cy, cyclophosphamide Mackall C, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 3000

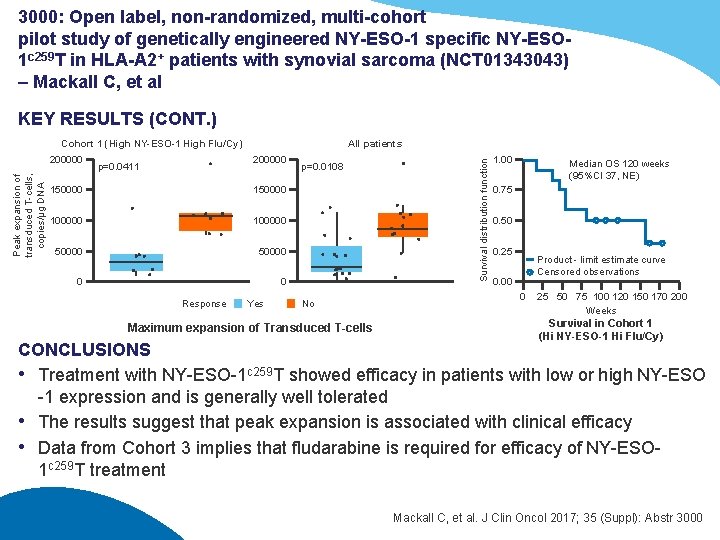
3000: Open label, non-randomized, multi-cohort pilot study of genetically engineered NY-ESO-1 specific NY-ESO 1 c 259 T in HLA-A 2+ patients with synovial sarcoma (NCT 01343043) – Mackall C, et al KEY RESULTS (CONT. ) Peak expansion of transduced T-cells, copies/μg DNA 200000 All patients 200000 p=0. 0411 150000 100000 50000 0 0 Response Yes p=0. 0108 No Maximum expansion of Transduced T-cells Survival distribution function Cohort 1 (High NY-ESO-1 High Flu/Cy) 1. 00 Median OS 120 weeks (95%CI 37, NE) 0. 75 0. 50 0. 25 Product - limit estimate curve Censored observations 0. 00 0 25 50 75 100 120 150 170 200 Weeks Survival in Cohort 1 (Hi NY-ESO-1 Hi Flu/Cy) CONCLUSIONS • Treatment with NY-ESO-1 c 259 T showed efficacy in patients with low or high NY-ESO -1 expression and is generally well tolerated • The results suggest that peak expansion is associated with clinical efficacy • Data from Cohort 3 implies that fludarabine is required for efficacy of NY-ESO 1 c 259 T treatment Mackall C, et al. J Clin Oncol 2017; 35 (Suppl): Abstr 3000
 Asco 2017 virtual meeting
Asco 2017 virtual meeting 2017 asco oncology practice conference
2017 asco oncology practice conference American epilepsy society annual meeting 2017
American epilepsy society annual meeting 2017 Da asco
Da asco Asco gu san francisco
Asco gu san francisco Asco coi
Asco coi Guias asco
Guias asco Gunter von minckwitz
Gunter von minckwitz June 2015 flacs exam answers
June 2015 flacs exam answers Annual theory meeting
Annual theory meeting Scts annual meeting
Scts annual meeting Aupha annual meeting
Aupha annual meeting How to run an annual general meeting
How to run an annual general meeting Cwemf
Cwemf Nrg oncology meeting 2017
Nrg oncology meeting 2017 American psychiatric association annual meeting 2020
American psychiatric association annual meeting 2020 Nrg oncology semi annual meeting 2018
Nrg oncology semi annual meeting 2018 Grand lodge of ky annual meeting
Grand lodge of ky annual meeting Aashto annual meeting 2015
Aashto annual meeting 2015 Informs annual meeting
Informs annual meeting Njdv
Njdv For todays meeting
For todays meeting Meeting objective
Meeting objective What is meeting and types of meeting
What is meeting and types of meeting What is meeting and types of meeting
What is meeting and types of meeting Job meeting bari 2017
Job meeting bari 2017 Nrg oncology meeting 2017
Nrg oncology meeting 2017 June too soon july stand by
June too soon july stand by June 22 to july 22
June 22 to july 22 June's journey
June's journey June canavan foundation
June canavan foundation 2019 june calendar
2019 june calendar Lenore hetrick pictures
Lenore hetrick pictures Nysedregents chemistry
Nysedregents chemistry Companies in june
Companies in june Welcome june blessings
Welcome june blessings June preschool newsletter
June preschool newsletter Battle of midway june 1942
Battle of midway june 1942 Irpwm june 2020
Irpwm june 2020 June holley
June holley June 2018 geometry regents answers
June 2018 geometry regents answers Good morning 1 june
Good morning 1 june June safety tips
June safety tips June 2010 physics regents answers
June 2010 physics regents answers June 6, 1944
June 6, 1944 Good morning please
Good morning please June's portfolio includes 177 shares
June's portfolio includes 177 shares June cheetah wegener
June cheetah wegener John 20:19-22
John 20:19-22 1 june children's day
1 june children's day