OASI ALK ASCO 2017 A cura di Filippo











- Slides: 27

OASI ALK ASCO 2017 A cura di Filippo de Marinis

Alectinib versus crizotinib in treatment-naive advanced ALK-positive non-small cell lung cancer (NSCLC): Primary results of the global phase III ALEX study Alice Tsang Shaw

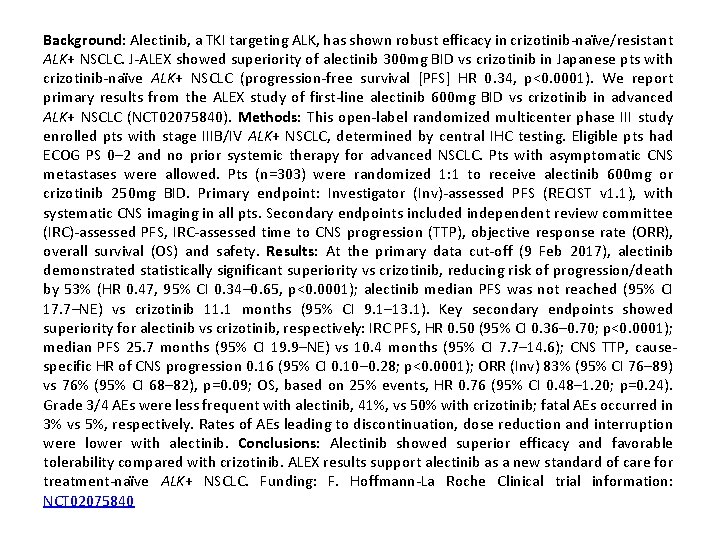
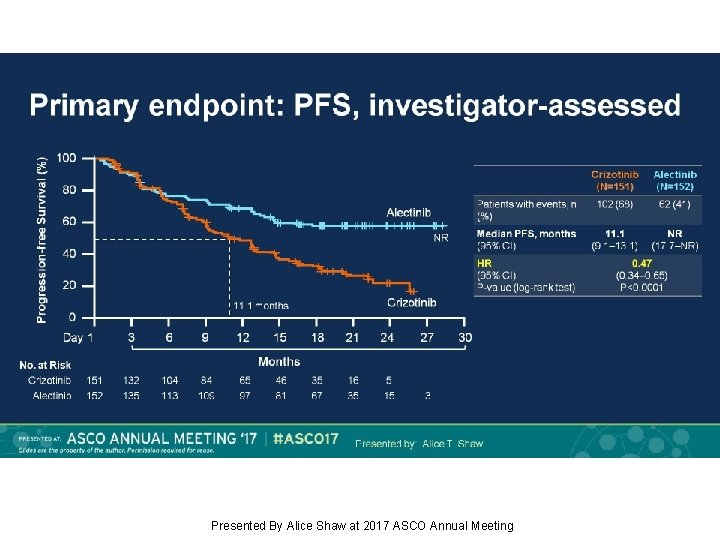
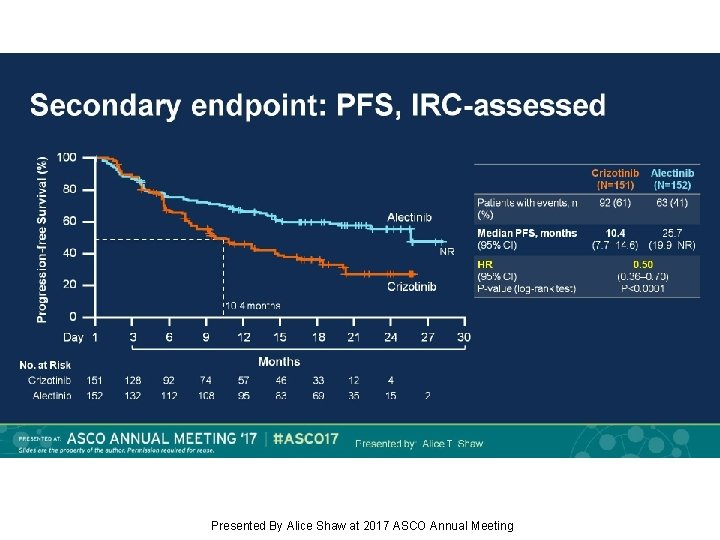
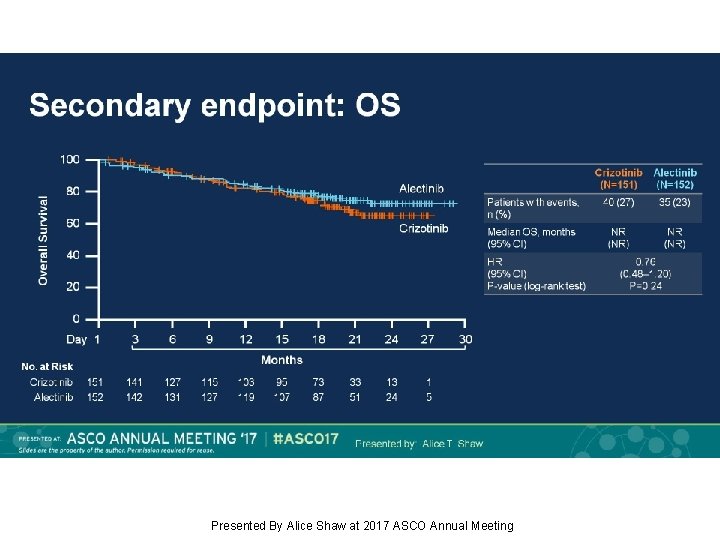
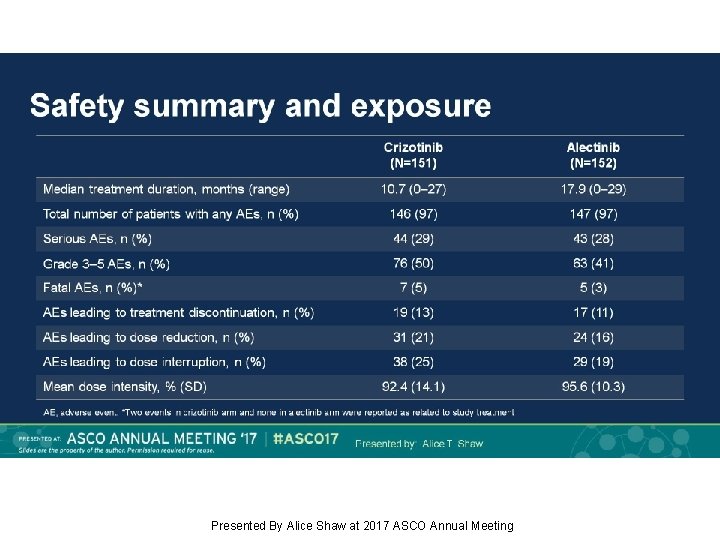
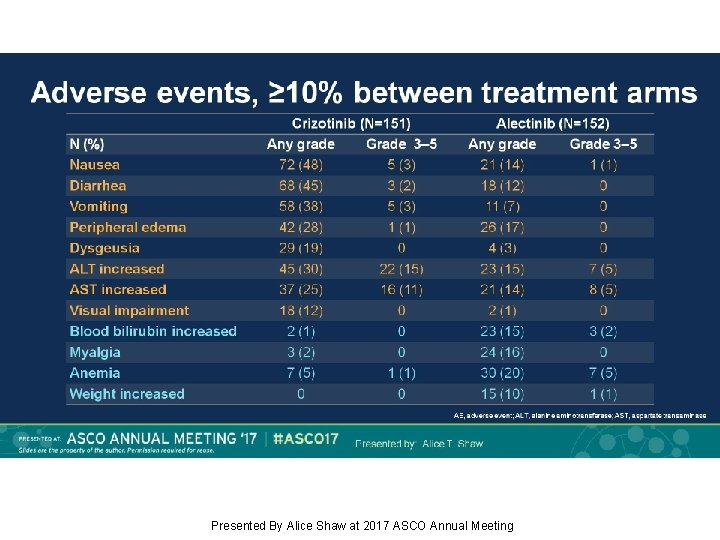
Background: Alectinib, a TKI targeting ALK, has shown robust efficacy in crizotinib-naïve/resistant ALK+ NSCLC. J-ALEX showed superiority of alectinib 300 mg BID vs crizotinib in Japanese pts with crizotinib-naïve ALK+ NSCLC (progression-free survival [PFS] HR 0. 34, p<0. 0001). We report primary results from the ALEX study of first-line alectinib 600 mg BID vs crizotinib in advanced ALK+ NSCLC (NCT 02075840). Methods: This open-label randomized multicenter phase III study enrolled pts with stage IIIB/IV ALK+ NSCLC, determined by central IHC testing. Eligible pts had ECOG PS 0– 2 and no prior systemic therapy for advanced NSCLC. Pts with asymptomatic CNS metastases were allowed. Pts (n=303) were randomized 1: 1 to receive alectinib 600 mg or crizotinib 250 mg BID. Primary endpoint: Investigator (Inv)-assessed PFS (RECIST v 1. 1), with systematic CNS imaging in all pts. Secondary endpoints included independent review committee (IRC)-assessed PFS, IRC-assessed time to CNS progression (TTP), objective response rate (ORR), overall survival (OS) and safety. Results: At the primary data cut-off (9 Feb 2017), alectinib demonstrated statistically significant superiority vs crizotinib, reducing risk of progression/death by 53% (HR 0. 47, 95% CI 0. 34– 0. 65, p<0. 0001); alectinib median PFS was not reached (95% CI 17. 7–NE) vs crizotinib 11. 1 months (95% CI 9. 1– 13. 1). Key secondary endpoints showed superiority for alectinib vs crizotinib, respectively: IRC PFS, HR 0. 50 (95% CI 0. 36– 0. 70; p<0. 0001); median PFS 25. 7 months (95% CI 19. 9–NE) vs 10. 4 months (95% CI 7. 7– 14. 6); CNS TTP, causespecific HR of CNS progression 0. 16 (95% CI 0. 10– 0. 28; p<0. 0001); ORR (Inv) 83% (95% CI 76– 89) vs 76% (95% CI 68– 82), p=0. 09; OS, based on 25% events, HR 0. 76 (95% CI 0. 48– 1. 20; p=0. 24). Grade 3/4 AEs were less frequent with alectinib, 41%, vs 50% with crizotinib; fatal AEs occurred in 3% vs 5%, respectively. Rates of AEs leading to discontinuation, dose reduction and interruption were lower with alectinib. Conclusions: Alectinib showed superior efficacy and favorable tolerability compared with crizotinib. ALEX results support alectinib as a new standard of care for treatment-naïve ALK+ NSCLC. Funding: F. Hoffmann-La Roche Clinical trial information: NCT 02075840

Alectinib vs crizotinib in treatment-naïve advanced ALK+ NSCLC: primary results of the global phase III ALEX study (LBA 9008) Presented By Alice Shaw at 2017 ASCO Annual Meeting

Slide 2 Presented By Alice Shaw at 2017 ASCO Annual Meeting

ALK rearrangement in NSCLC Presented By Alice Shaw at 2017 ASCO Annual Meeting

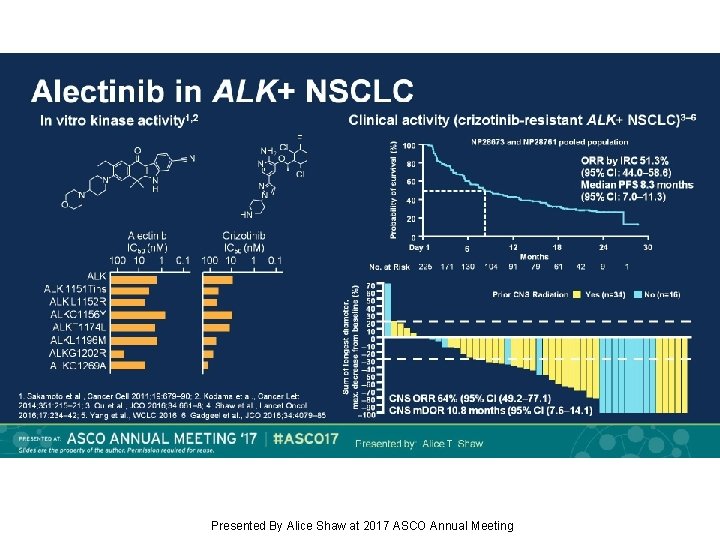
Alectinib in ALK+ NSCLC Presented By Alice Shaw at 2017 ASCO Annual Meeting

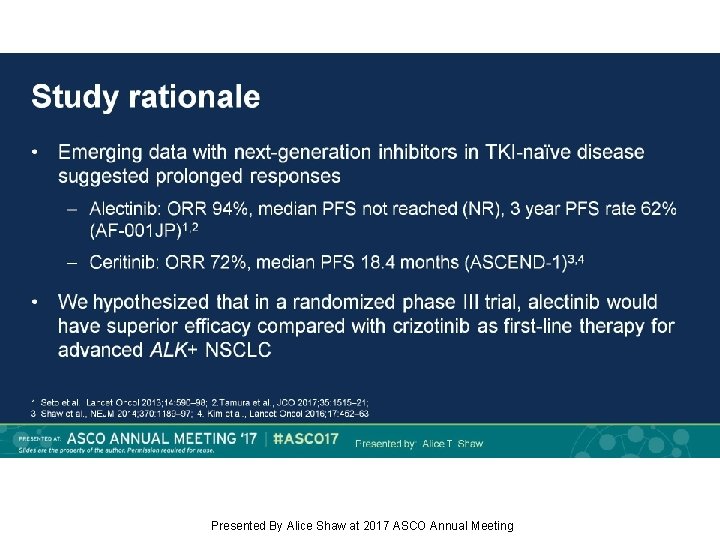
Study rationale Presented By Alice Shaw at 2017 ASCO Annual Meeting

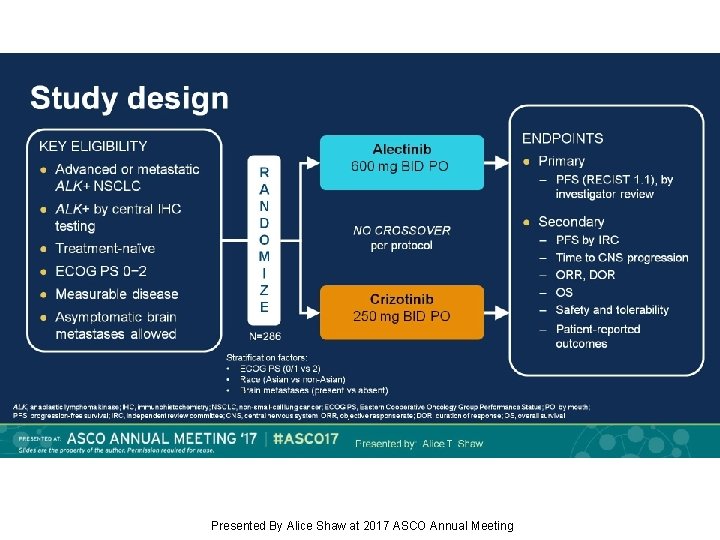
Study design Presented By Alice Shaw at 2017 ASCO Annual Meeting

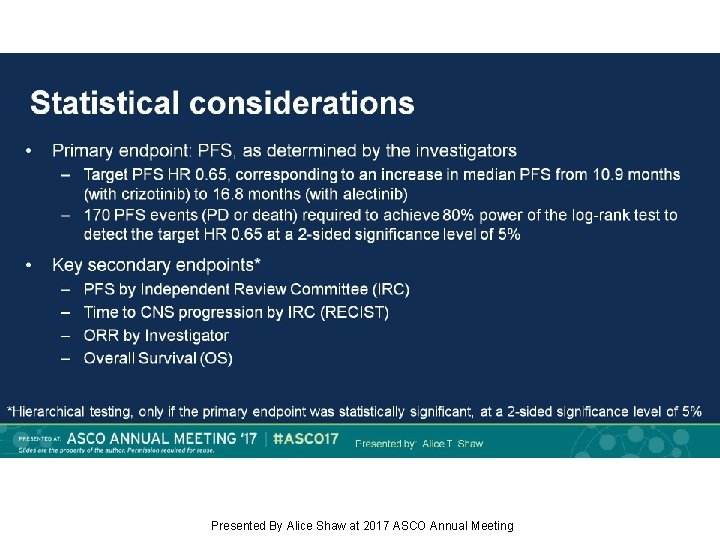
Statistical considerations Presented By Alice Shaw at 2017 ASCO Annual Meeting

Study conduct Presented By Alice Shaw at 2017 ASCO Annual Meeting

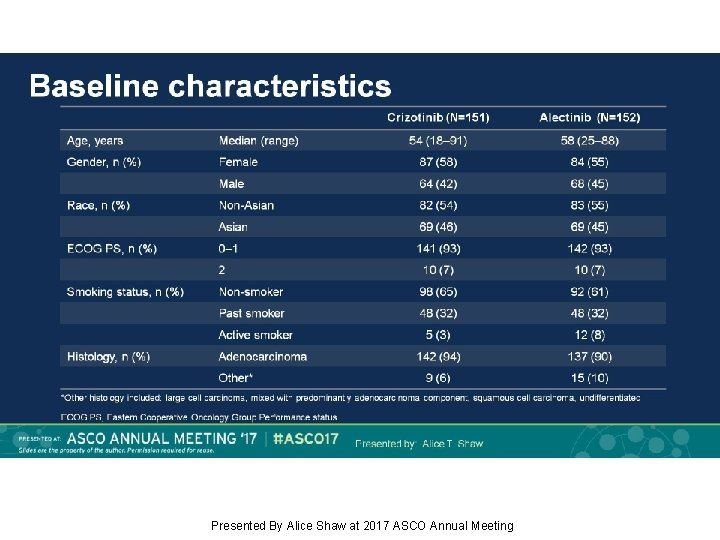
Baseline characteristics Presented By Alice Shaw at 2017 ASCO Annual Meeting

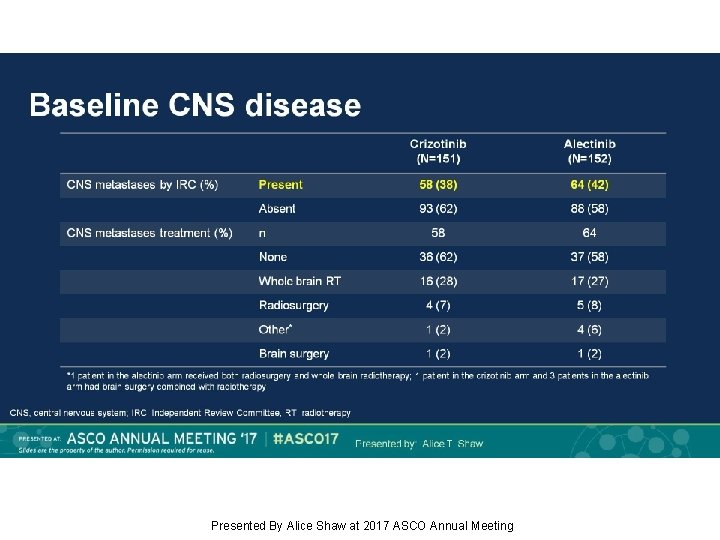
Baseline CNS disease Presented By Alice Shaw at 2017 ASCO Annual Meeting

Primary endpoint: PFS, investigator-assessed Presented By Alice Shaw at 2017 ASCO Annual Meeting

Secondary endpoint: PFS, IRC-assessed Presented By Alice Shaw at 2017 ASCO Annual Meeting

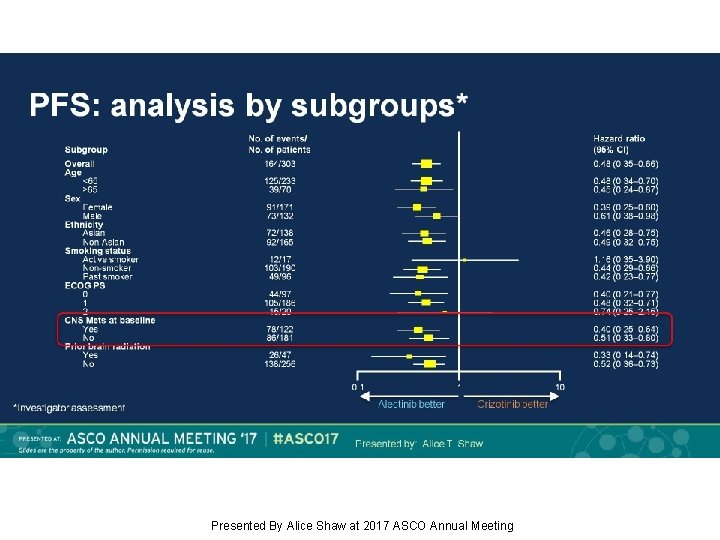
PFS: analysis by subgroups* Presented By Alice Shaw at 2017 ASCO Annual Meeting

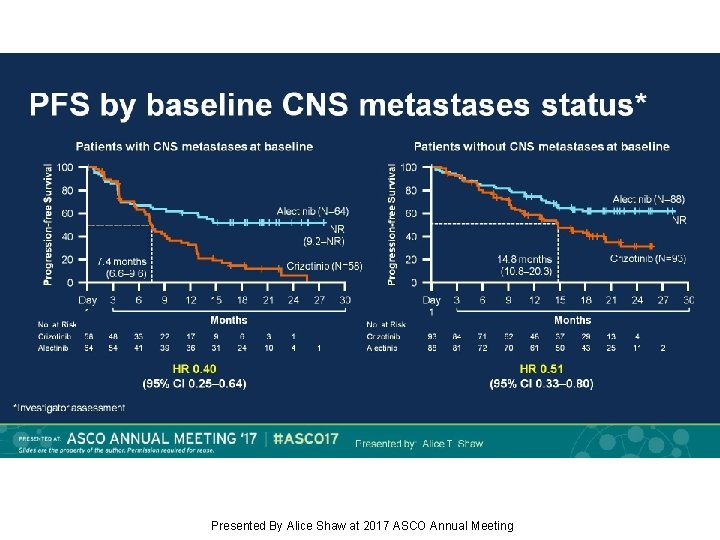
PFS by baseline CNS metastases status* Presented By Alice Shaw at 2017 ASCO Annual Meeting

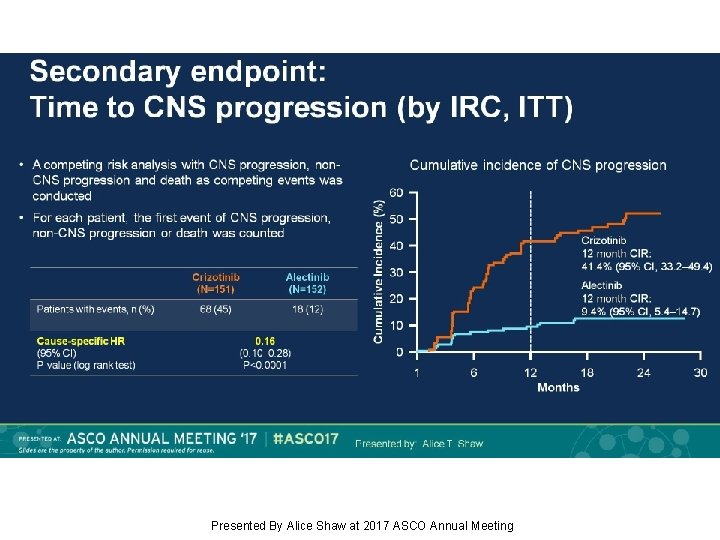
Secondary endpoint: Time to CNS progression (by IRC, ITT) Presented By Alice Shaw at 2017 ASCO Annual Meeting

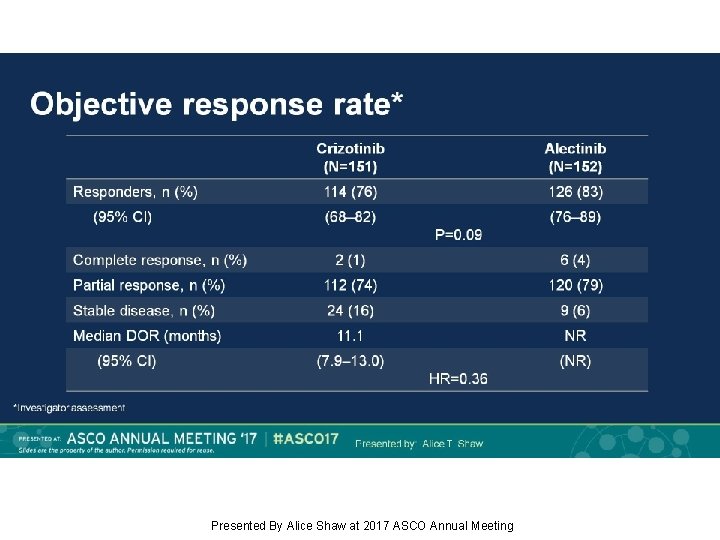
Objective response rate* Presented By Alice Shaw at 2017 ASCO Annual Meeting

CNS objective response rate* Presented By Alice Shaw at 2017 ASCO Annual Meeting

Secondary endpoint: OS Presented By Alice Shaw at 2017 ASCO Annual Meeting

Safety summary and exposure Presented By Alice Shaw at 2017 ASCO Annual Meeting

Adverse events, ≥ 10% between treatment arms Presented By Alice Shaw at 2017 ASCO Annual Meeting

Summary Presented By Alice Shaw at 2017 ASCO Annual Meeting

Conclusions Presented By Alice Shaw at 2017 ASCO Annual Meeting

Acknowledgments Presented By Alice Shaw at 2017 ASCO Annual Meeting

Slide 24 Presented By Alice Shaw at 2017 ASCO Annual Meeting
 2017 asco oncology practice conference
2017 asco oncology practice conference Asco 2017 virtual meeting
Asco 2017 virtual meeting Cave del praello
Cave del praello Oasi care bundle information leaflet
Oasi care bundle information leaflet Kitchen safety
Kitchen safety Asco coi
Asco coi Diferencia entre centinela y atalaya
Diferencia entre centinela y atalaya Asco
Asco ¿cuál es tu restaurante favorito?
¿cuál es tu restaurante favorito? Asco gu san francisco
Asco gu san francisco Biblioteka alk
Biblioteka alk Alk biblioteka katalog
Alk biblioteka katalog Alectinib forum
Alectinib forum Segi 15 tidak beraturan
Segi 15 tidak beraturan Alk biblioteka
Alk biblioteka All'automobile da corsa poesia
All'automobile da corsa poesia Inferno canto 8 parafrasi
Inferno canto 8 parafrasi Oratoria deliberativa
Oratoria deliberativa Marta marchioro
Marta marchioro Filippo brunelleschi perspectiva
Filippo brunelleschi perspectiva Filippo turato
Filippo turato Filippo tomasello
Filippo tomasello Ic de filippo sant'egidio
Ic de filippo sant'egidio Laurent di filippo
Laurent di filippo Ic de filippo sant'egidio
Ic de filippo sant'egidio Istituto comprensivo losapio san filippo neri
Istituto comprensivo losapio san filippo neri Prova di perthes
Prova di perthes Circolo didattico eduardo de filippo
Circolo didattico eduardo de filippo