NSCLC KOL Insight Report Overview NSCLC KOL Insight











- Slides: 16

NSCLC: KOL Insight

Report Overview NSCLC: KOL Insight Provides balanced and independent expert opinions about marketed and late-stage targeted therapies in the Non-Small Cell Lung Cancer market with an emphasis on current and future treatment pathways. *Includes an addendum focussed on the failure of the Check. Mate 026 trial of Opdivo and its likely implications, based on additional interviews conducted during the week after this news broke. Qualitative Insights: In-depth interviews with the foremost key opinion leaders answering critical business questions. Research Focus: Key battlegrounds for market share and the factors affecting current and future product positioning. Updates: Analysts continually monitor the market and frequently re-engage with KOLs ensuring insights remain current and relevant.

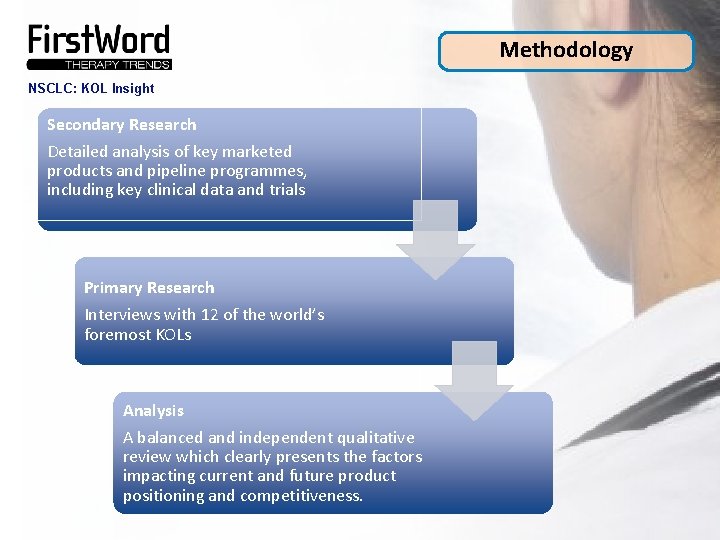
Methodology NSCLC: KOL Insight Secondary Research Detailed analysis of key marketed products and pipeline programmes, including key clinical data and trials Primary Research Interviews with 12 of the world’s foremost KOLs Analysis A balanced and independent qualitative review which clearly presents the factors impacting current and future product positioning and competitiveness.

Research Objectives NSCLC: KOL Insight • Analysis of product attributes which confer preferred status • Product perception by the medical community • Clinical trials with greatest potential to impact prescribing trends • Therapy attributes needed to become the treatment of choice • The future role of current and late-stage pipeline therapies • Assessment of current and pipeline products and their likely impact • Mapping the future evolution of the treatment landscape

Business Questions NSCLC: KOL Insight § § § § § What are KOLs’ views on the design of the Check. Mate-026 trial of Opdivo and the likely implications of its failure? How will Opdivo be used in the future? How do KOLs think Keytruda and Opdivo compare in terms of efficacy and safety? Are KOLs optimistic regarding the Keytruda Phase III PEARLS trial in the adjuvant setting? Is the ARCTIC trial of durvalumab as a third-line agent viewed favourably by KOLs? What factors will guide patient selection for Yervoy/Opdivo combination therapy? What factors could help avelumab compete? What are KOLs’ expectations regarding Tagrisso’s first-line FLAURA trial? Will rociletinib be able to compete with Tagrisso? Why? /Why not? How is Xalkori currently used and how do KOLs envisage its use in the future? Is Zykadia’s position as second-line standard-of-care for ALK-positive NSCLC under threat in the US? How do experts perceive Alecensa’s ALEX trial? What could help Avastin maintain a presence in a rapidly evolving market? What factors may hinder the uptake of Vargatef? How do KOLs expect Cyramza to fare in the EU market? According to KOLs, what is limiting the uptake of Portrazza? Where in the treatment paradigm could abemaciclib be used?

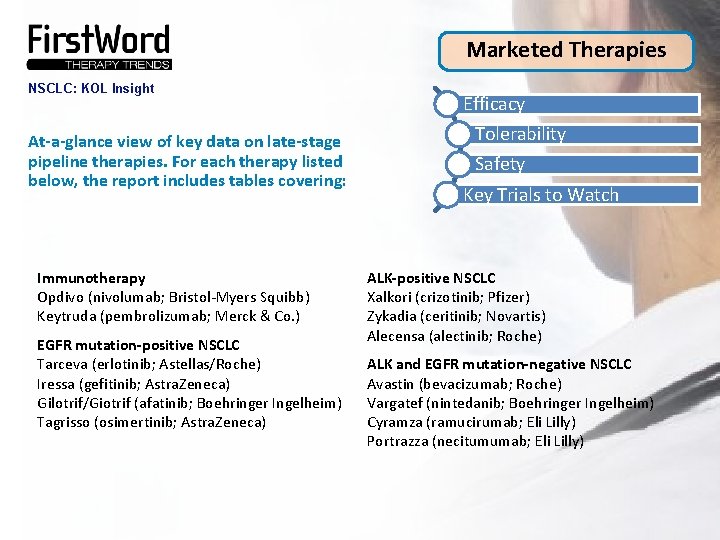
Marketed Therapies NSCLC: KOL Insight At-a-glance view of key data on late-stage pipeline therapies. For each therapy listed below, the report includes tables covering: Immunotherapy Opdivo (nivolumab; Bristol-Myers Squibb) Keytruda (pembrolizumab; Merck & Co. ) EGFR mutation-positive NSCLC Tarceva (erlotinib; Astellas/Roche) Iressa (gefitinib; Astra. Zeneca) Gilotrif/Giotrif (afatinib; Boehringer Ingelheim) Tagrisso (osimertinib; Astra. Zeneca) Efficacy Tolerability Safety Key Trials to Watch ALK-positive NSCLC Xalkori (crizotinib; Pfizer) Zykadia (ceritinib; Novartis) Alecensa (alectinib; Roche) ALK and EGFR mutation-negative NSCLC Avastin (bevacizumab; Roche) Vargatef (nintedanib; Boehringer Ingelheim) Cyramza (ramucirumab; Eli Lilly) Portrazza (necitumumab; Eli Lilly)

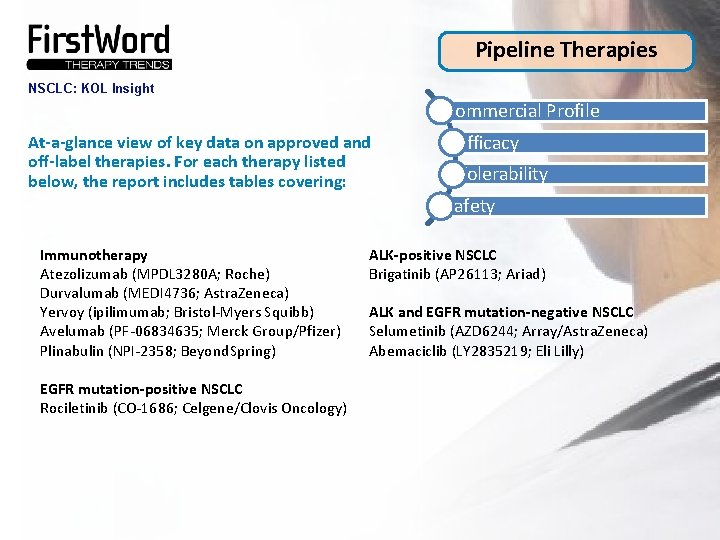
Pipeline Therapies NSCLC: KOL Insight Commercial Profile At-a-glance view of key data on approved and off-label therapies. For each therapy listed below, the report includes tables covering: Efficacy Tolerability Safety Immunotherapy Atezolizumab (MPDL 3280 A; Roche) Durvalumab (MEDI 4736; Astra. Zeneca) Yervoy (ipilimumab; Bristol-Myers Squibb) Avelumab (PF-06834635; Merck Group/Pfizer) Plinabulin (NPI-2358; Beyond. Spring) EGFR mutation-positive NSCLC Rociletinib (CO-1686; Celgene/Clovis Oncology) ALK-positive NSCLC Brigatinib (AP 26113; Ariad) ALK and EGFR mutation-negative NSCLC Selumetinib (AZD 6244; Array/Astra. Zeneca) Abemaciclib (LY 2835219; Eli Lilly)

KOLs Interviewed NSCLC: KOL Insight KOLs from North America Catherine Azar; Clinical Associate Professor of Medicine, Hematology/Oncology Department, University of Arizona, Tucson, AZ Paul Bunn; Distinguished Professor, Division of Medical Oncology, University of Colorado, Boulder, CO Renata Ferrarotto; Assistant Professor, Department of Thoracic/Head and Neck Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX Edward Garon; MD, Associate Clinical Professor, Thoratic Oncology Program, Department of Hematology and Oncology, David Geffen School of Medicine, UCLA, Los Angeles, CA Jared Weiss; Assistant Professor, School of Medicine, University of North Carolina at Chapel Hill, Clinical Research, Thoracic Oncology Program, Chapel Hill, NC Howard West; Medical Director, Thoracic Oncology Program, Swedish Cancer Institute; President & CEO, Global Resource for Advancing Cancer Education (GRACE), Seattle, WA Anonymous KOL; Associate Professor at a major US Medical School KOLs from EU Qamar Ghafoor; Consultant Clinical Oncologist at University Hospital Birmingham, UK Jose I. Mayordomo; Professor, Medical Oncologist, Medical Oncology at the University Hospital of Zaragoza, Spain/Professor, Division of Medical Oncology, University of Colorado School of Medicine, Denver, CO, USA Marie Wislez; Consultant, Tenon Hospital, Paris, France Anonymous German KOL (x 2)

Table of Contents NSCLC: KOL Insight

Table of Contents NSCLC: KOL Insight

Table of Contents NSCLC: KOL Insight

Sample Page(s) NSCLC: KOL Insight

Sample Page(s) NSCLC: KOL Insight

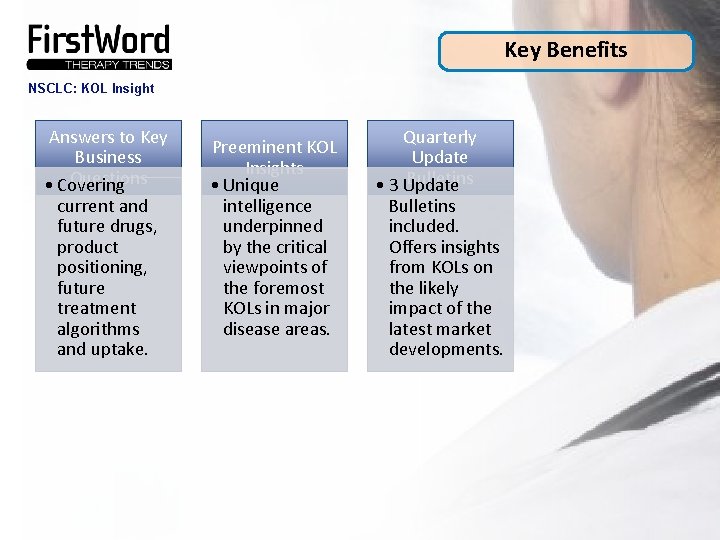
Key Benefits NSCLC: KOL Insight Answers to Key Business Questions • Covering current and future drugs, product positioning, future treatment algorithms and uptake. Preeminent KOL Insights • Unique intelligence underpinned by the critical viewpoints of the foremost KOLs in major disease areas. Quarterly Update Bulletins • 3 Update Bulletins included. Offers insights from KOLs on the likely impact of the latest market developments.

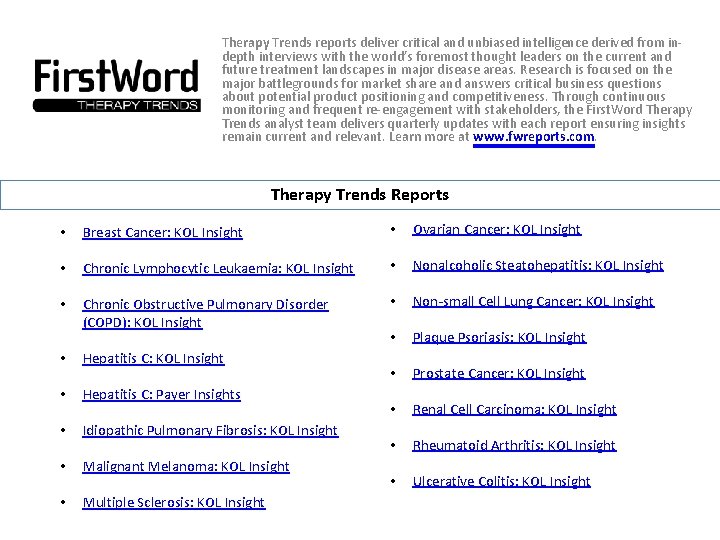
Therapy Trends reports deliver critical and unbiased intelligence derived from indepth interviews with the world’s foremost thought leaders on the current and future treatment landscapes in major disease areas. Research is focused on the major battlegrounds for market share and answers critical business questions about potential product positioning and competitiveness. Through continuous monitoring and frequent re-engagement with stakeholders, the First. Word Therapy Trends analyst team delivers quarterly updates with each report ensuring insights remain current and relevant. Learn more at www. fwreports. com. Therapy Trends Reports • Breast Cancer: KOL Insight • Ovarian Cancer: KOL Insight • Chronic Lymphocytic Leukaemia: KOL Insight • Nonalcoholic Steatohepatitis: KOL Insight • Chronic Obstructive Pulmonary Disorder (COPD): KOL Insight • Non-small Cell Lung Cancer: KOL Insight • Plaque Psoriasis: KOL Insight • Prostate Cancer: KOL Insight • Renal Cell Carcinoma: KOL Insight • Rheumatoid Arthritis: KOL Insight • Ulcerative Colitis: KOL Insight • Hepatitis C: Payer Insights • Idiopathic Pulmonary Fibrosis: KOL Insight • Malignant Melanoma: KOL Insight • Multiple Sclerosis: KOL Insight

Unique insight into current and future pharma market dynamics through quantitative surveys with physicians, providing essential data in major disease areas and on key industry issues. A personalised and comprehensive intelligence service delivering up-to-theminute pharma news, insight, analysis, and expert views of importance to your company's success. Critical and unbiased intelligence derived from in-depth interviews with the world’s foremost thought leaders on the current and future treatment landscapes in major disease areas. Reports include three quarterly updates to ensure insights remain current. A personalised and comprehensive intelligence service reporting on the latest news and developments for the medical technology and diagnostic industries. Unbiased and concise analysis based on interviews with leading industry experts on important trends and challenging issues affecting the pharma industry today. First. Word delivers timely, need-to-know intelligence about your products, your competitors and your markets.
 Adjuvant nsclc
Adjuvant nsclc Adjuvant nsclc
Adjuvant nsclc Nsclc
Nsclc Difference between progress report and status report
Difference between progress report and status report Partial report technique
Partial report technique Brakial arter nerede
Brakial arter nerede Kompartman sendromu fasyotomi
Kompartman sendromu fasyotomi Kol nidrei text
Kol nidrei text Kol gücünden makine gücüne geçiş
Kol gücünden makine gücüne geçiş Respiratorisk acidose kol
Respiratorisk acidose kol Barak kol
Barak kol Dirsek fleksörleri
Dirsek fleksörleri Henle kulpu inen kol
Henle kulpu inen kol Pr��ce kol��n
Pr��ce kol��n Postfænomenologi
Postfænomenologi Kolun ön yüz kasları
Kolun ön yüz kasları Kol askısıyla önkol bilek ve el tespiti
Kol askısıyla önkol bilek ve el tespiti