APPLICATIONS OF DEXMEDETOMIDINE IN PEDIATRIC PROCEDURAL SEDATION GOALS










- Slides: 43

APPLICATIONS OF DEXMEDETOMIDINE IN PEDIATRIC PROCEDURAL SEDATION

GOALS • Understand the pharmacology, physiology, and clinical properties of dexmedetomidine • Review clinical experience with dexmedetomidine for pediatric procedural sedation • Adverse Events/Safety Profile • Coadministrations • Alternative administration methods • Discuss practical issues related to use

BACKGROUND • Despite recognition of sedation importance, few agent developments in recent past • Significant issues with some current agents • • • Opiate/benzodiazepine – tolerance, efficacy Chloral hydrate - predictability Pentobarbital – agitation, duration Propofol – limited access in some jurisdictions Ketamine – emergence reactions, tolerance • 2 -adrenoreceptor agonism

BACKGROUND 2 RECPTOR AGONISTS • Prototype agent is clonidine • More recent applications in clinical practice • • Sedation Behavior disorders (ADHD) Drug withdrawal Hypertension • Problem – hypotension, oral = slow • Solution – 2 nd generation - 2 specificity

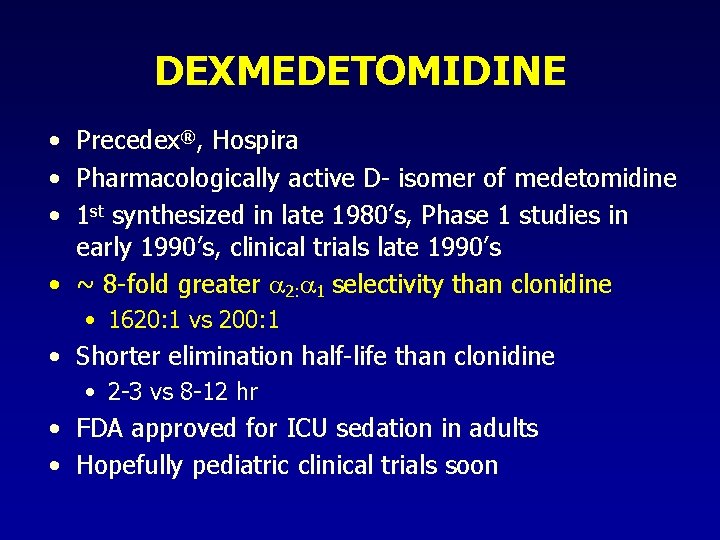
DEXMEDETOMIDINE • Precedex®, Hospira • Pharmacologically active D- isomer of medetomidine • 1 st synthesized in late 1980’s, Phase 1 studies in early 1990’s, clinical trials late 1990’s • ~ 8 -fold greater 2: 1 selectivity than clonidine • 1620: 1 vs 200: 1 • Shorter elimination half-life than clonidine • 2 -3 vs 8 -12 hr • FDA approved for ICU sedation in adults • Hopefully pediatric clinical trials soon

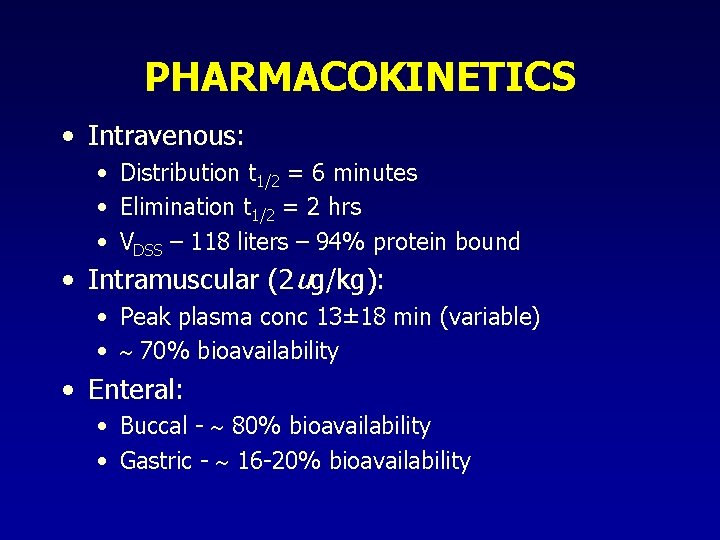
PHARMACOKINETICS • Intravenous: • Distribution t 1/2 = 6 minutes • Elimination t 1/2 = 2 hrs • VDSS – 118 liters – 94% protein bound • Intramuscular (2 ug/kg): • Peak plasma conc 13± 18 min (variable) • 70% bioavailability • Enteral: • Buccal - 80% bioavailability • Gastric - 16 -20% bioavailability

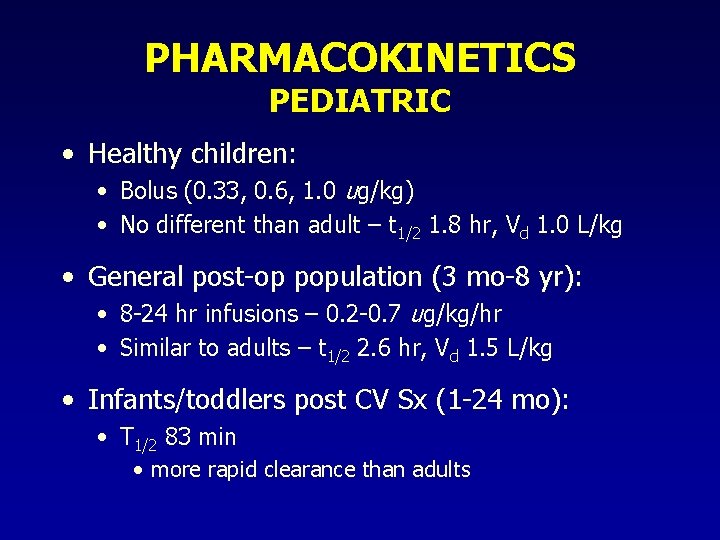
PHARMACOKINETICS PEDIATRIC • Healthy children: • Bolus (0. 33, 0. 6, 1. 0 ug/kg) • No different than adult – t 1/2 1. 8 hr, Vd 1. 0 L/kg • General post-op population (3 mo-8 yr): • 8 -24 hr infusions – 0. 2 -0. 7 ug/kg/hr • Similar to adults – t 1/2 2. 6 hr, Vd 1. 5 L/kg • Infants/toddlers post CV Sx (1 -24 mo): • T 1/2 83 min • more rapid clearance than adults

METABOLISM • Almost 100% biotransformation • Glucuronidation • Cytochrome P 450 mediated • Metabolites all inactive – urinary elimination • Significant t 1/2 in hepatic failure (7. 5 hr) • <1% excreted as unchanged • No significant effect of renal impairment

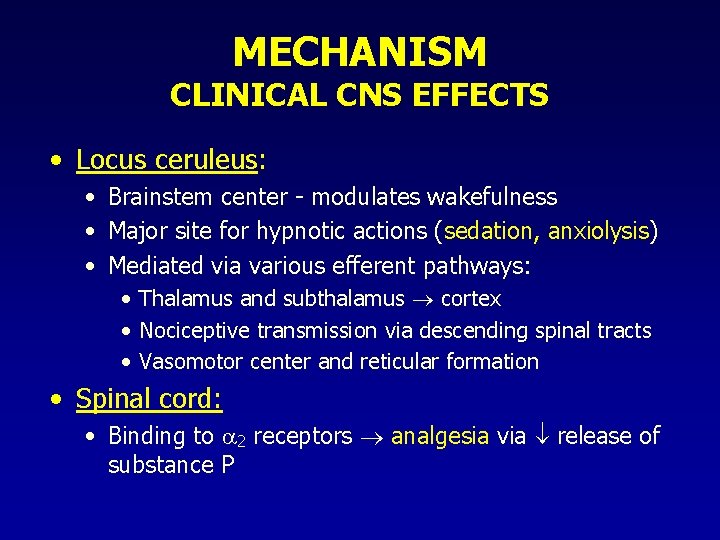
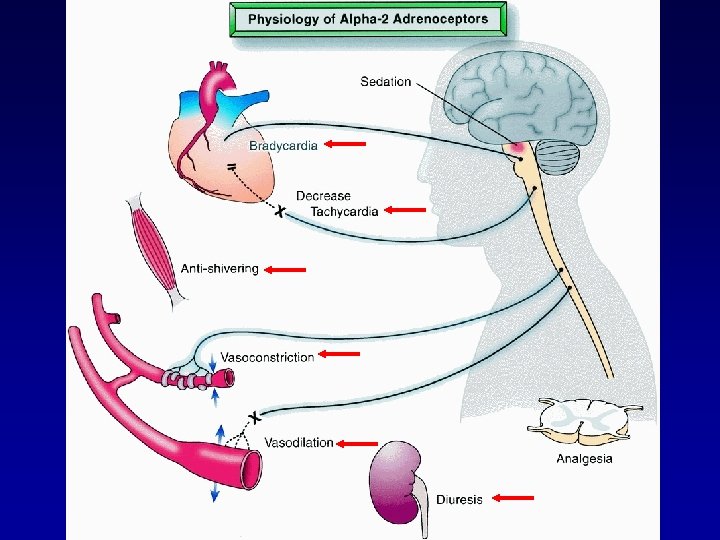
MECHANISM CLINICAL CNS EFFECTS • Locus ceruleus: • Brainstem center - modulates wakefulness • Major site for hypnotic actions (sedation, anxiolysis) • Mediated via various efferent pathways: • Thalamus and subthalamus cortex • Nociceptive transmission via descending spinal tracts • Vasomotor center and reticular formation • Spinal cord: • Binding to 2 receptors analgesia via release of substance P

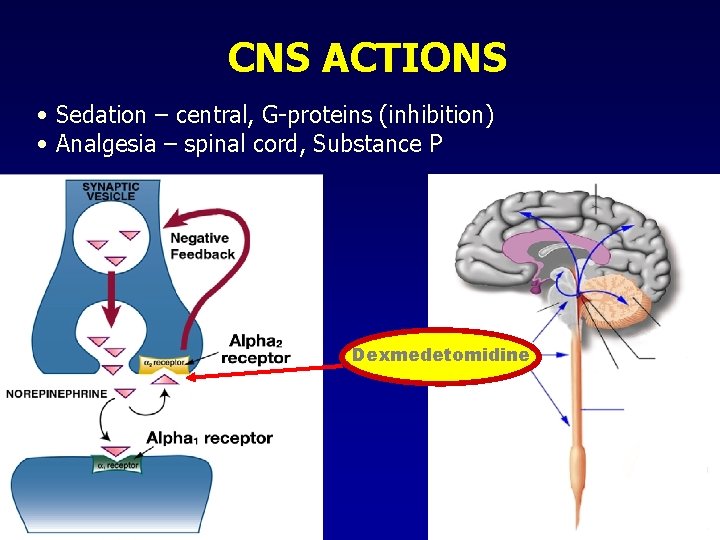
CNS ACTIONS • Sedation – central, G-proteins (inhibition) • Analgesia – spinal cord, Substance P Dexmedetomidine

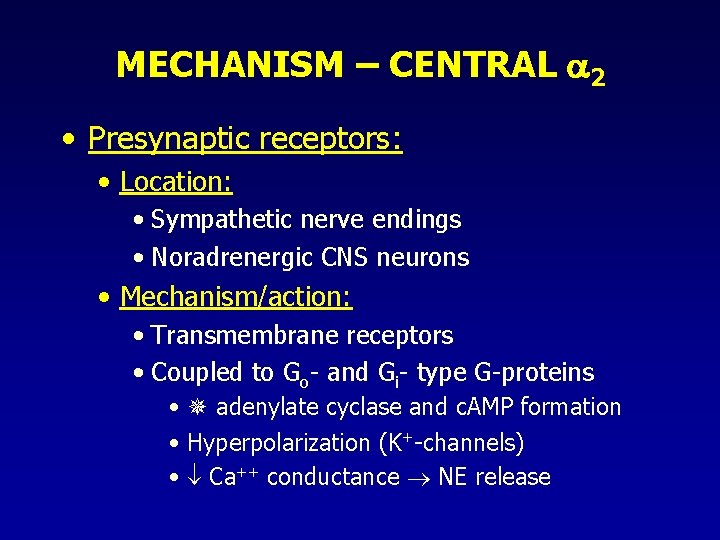
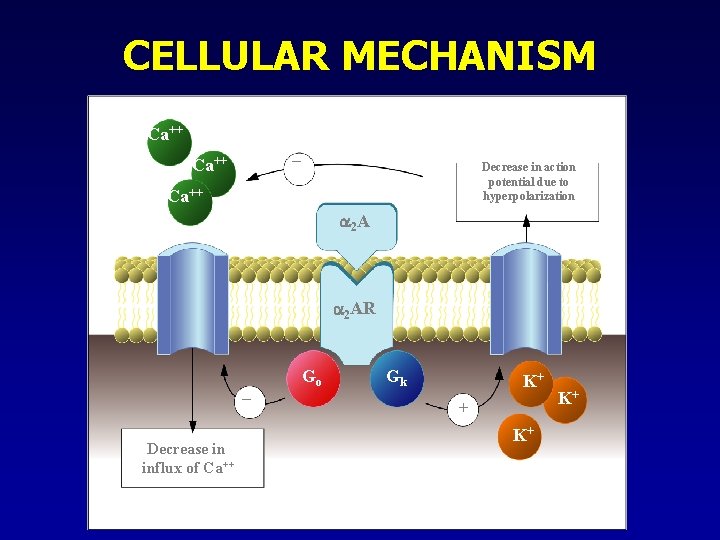
MECHANISM – CENTRAL 2 • Presynaptic receptors: • Location: • Sympathetic nerve endings • Noradrenergic CNS neurons • Mechanism/action: • Transmembrane receptors • Coupled to Go- and Gi- type G-proteins • adenylate cyclase and c. AMP formation • Hyperpolarization (K+-channels) • Ca++ conductance NE release

CELLULAR MECHANISM Ca++ – Ca++ Decrease in action potential due to hyperpolarization Ca++ 2 A 2 AR – Decrease in influx of Ca++ Go Gk K+ + K+ K+

NON-CNS EFFECTS • Hypertension: • peripheral 1 -agonism • Bradycardia/hypotension: • Sympathetic inhibition - medullary VMC • shivering: • Diuresis: • renin, vasopressin; ANP


RESPIRATORY EFFECTS • Promoted as having minimal respiratory depressing effects • 0. 17% incidence on monogram • Most data suggests Sa. O 2 and Pa. CO 2 unaffected • Numerous reports during spontaneous ventilation

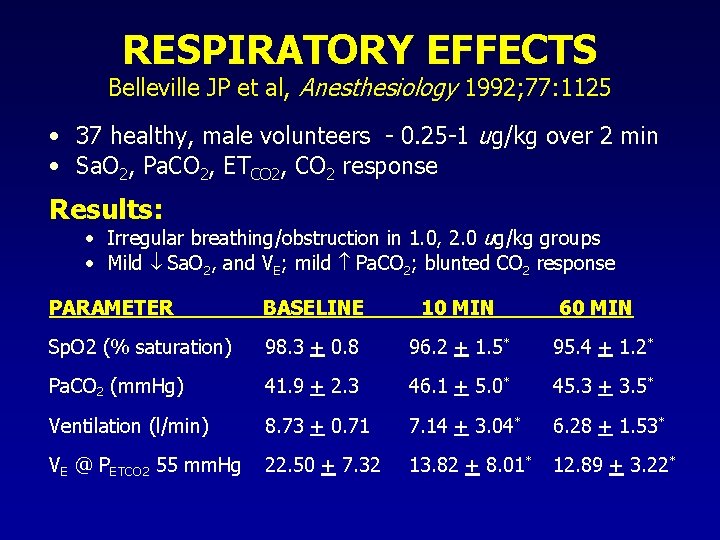
RESPIRATORY EFFECTS Belleville JP et al, Anesthesiology 1992; 77: 1125 • 37 healthy, male volunteers - 0. 25 -1 ug/kg over 2 min • Sa. O 2, Pa. CO 2, ETCO 2, CO 2 response Results: • Irregular breathing/obstruction in 1. 0, 2. 0 ug/kg groups • Mild Sa. O 2, and VE; mild Pa. CO 2; blunted CO 2 response PARAMETER BASELINE 10 MIN 60 MIN Sp. O 2 (% saturation) 98. 3 + 0. 8 96. 2 + 1. 5* 95. 4 + 1. 2* Pa. CO 2 (mm. Hg) 41. 9 + 2. 3 46. 1 + 5. 0* 45. 3 + 3. 5* Ventilation (l/min) 8. 73 + 0. 71 7. 14 + 3. 04* 6. 28 + 1. 53* VE @ PETCO 2 55 mm. Hg 22. 50 + 7. 32 13. 82 + 8. 01* 12. 89 + 3. 22*

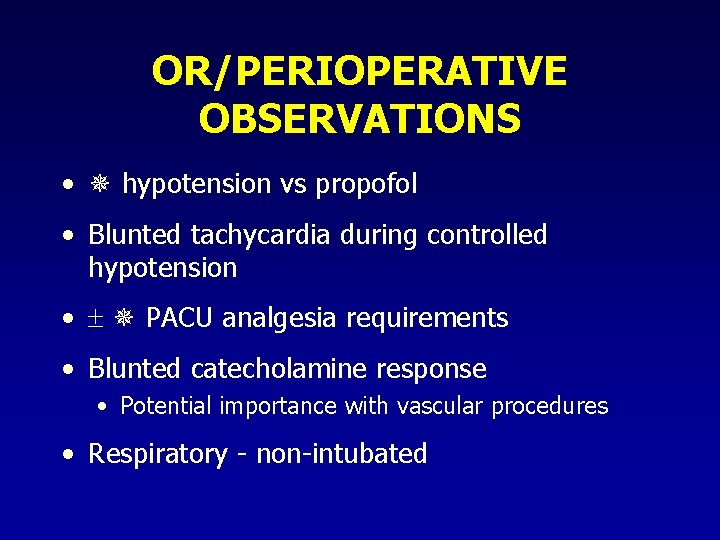
OR/PERIOPERATIVE OBSERVATIONS • hypotension vs propofol • Blunted tachycardia during controlled hypotension • PACU analgesia requirements • Blunted catecholamine response • Potential importance with vascular procedures • Respiratory - non-intubated

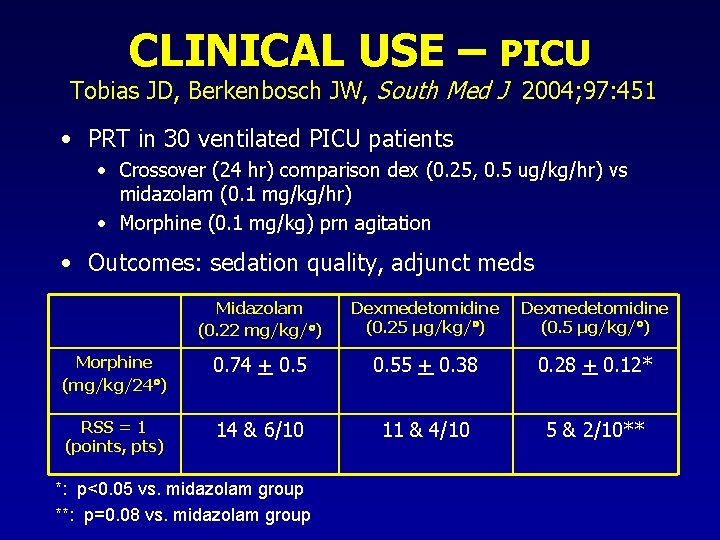
CLINICAL USE – PICU Tobias JD, Berkenbosch JW, South Med J 2004; 97: 451 • PRT in 30 ventilated PICU patients • Crossover (24 hr) comparison dex (0. 25, 0. 5 ug/kg/hr) vs midazolam (0. 1 mg/kg/hr) • Morphine (0. 1 mg/kg) prn agitation • Outcomes: sedation quality, adjunct meds Midazolam (0. 22 mg/kg/ ) Dexmedetomidine (0. 25 µg/kg/ ) Dexmedetomidine (0. 5 µg/kg/ ) Morphine (mg/kg/24 ) 0. 74 + 0. 55 + 0. 38 0. 28 + 0. 12* RSS = 1 (points, pts) 14 & 6/10 11 & 4/10 5 & 2/10** *: p<0. 05 vs. midazolam group **: p=0. 08 vs. midazolam group

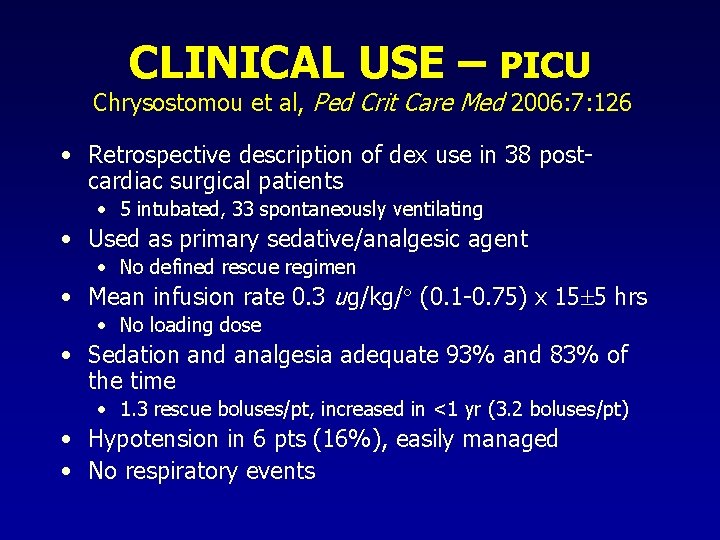
CLINICAL USE – PICU Chrysostomou et al, Ped Crit Care Med 2006: 7: 126 • Retrospective description of dex use in 38 postcardiac surgical patients • 5 intubated, 33 spontaneously ventilating • Used as primary sedative/analgesic agent • No defined rescue regimen • Mean infusion rate 0. 3 ug/kg/ (0. 1 -0. 75) x 15 5 hrs • No loading dose • Sedation and analgesia adequate 93% and 83% of the time • 1. 3 rescue boluses/pt, increased in <1 yr (3. 2 boluses/pt) • Hypotension in 6 pts (16%), easily managed • No respiratory events

CLINICAL USE – PICU Buck et al, Pharmacotherapy 2008: 7: 51 • Prospective, observational series of dex in 17 PICU patients (20 courses) • cardiac surgical (13), medical (3), other surg (1) • Dose range 0. 2 -0. 7 ug/kg/ x 32 21 hr • No loading dose • Primary agent in 15, adjunct in 5 (failed conv) • periextubation agent in 13 - all successful • No reported significant cardiovascular events

ICU OBSERVATIONS • Limited available data • Peds doses may be slightly higher, esp infants • Parent satisfaction high • Lighter but less agitated • sedation/recovery-related “wooziness” • Appears useful in non-intubated pts • Effective bridge through extubation • Not necessarily 1 st line • reserve for difficult, long-term • Analgesic effects probably not insignificant

PROCEDURAL SEDATION • Most recently reported application but more published information compared with ICU • Expansion developed based on confirmation of limited resp depression • Nichols DP, et al Pediatr Anaesth 2005; 15: 199 • • Sedation of 5 children failing chloral hydrate/midazolam Dex bolus (0. 8 0. 4 ug/kg) over 10 min, gtt 0. 6 ug/kg/hr Procedures completed Modest HR, BP; no significant respiratory effects

PROCEDURAL SEDATION Berkenbosch JW, Pediatr Crit Care Med 2005; 6: 435 • First reported prospective series • non-invasive procedures • Candidates: • >4 y. o. • Previous chloral hydrate failure/poor candidate • Rescue from failed sedation • Induction bolus: 0. 5 ug/kg over 5 min • Maintenance: started at 0. 5 ug/kg/hr - titrate • Monitor - Physiologic - Effectiveness - Recovery-related behavior

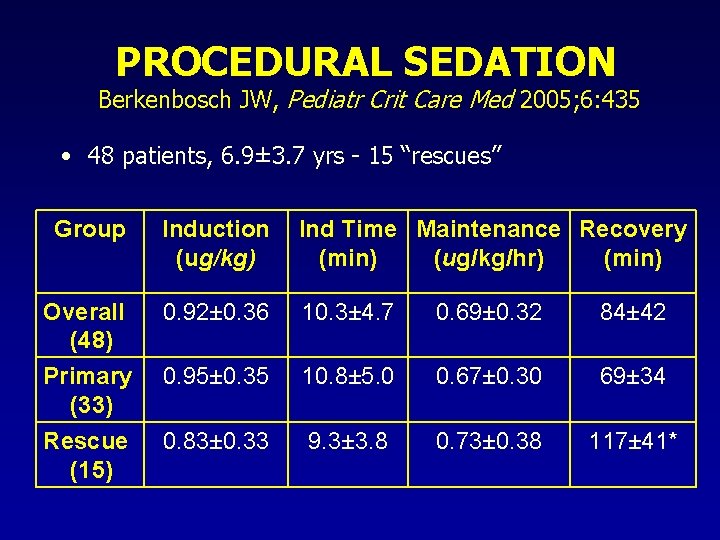
PROCEDURAL SEDATION Berkenbosch JW, Pediatr Crit Care Med 2005; 6: 435 • 48 patients, 6. 9± 3. 7 yrs - 15 “rescues” Group Induction (ug/kg) Ind Time Maintenance Recovery (min) (ug/kg/hr) (min) Overall (48) 0. 92± 0. 36 10. 3± 4. 7 0. 69± 0. 32 84± 42 Primary (33) Rescue (15) 0. 95± 0. 35 10. 8± 5. 0 0. 67± 0. 30 69± 34 0. 83± 0. 33 9. 3± 3. 8 0. 73± 0. 38 117± 41*

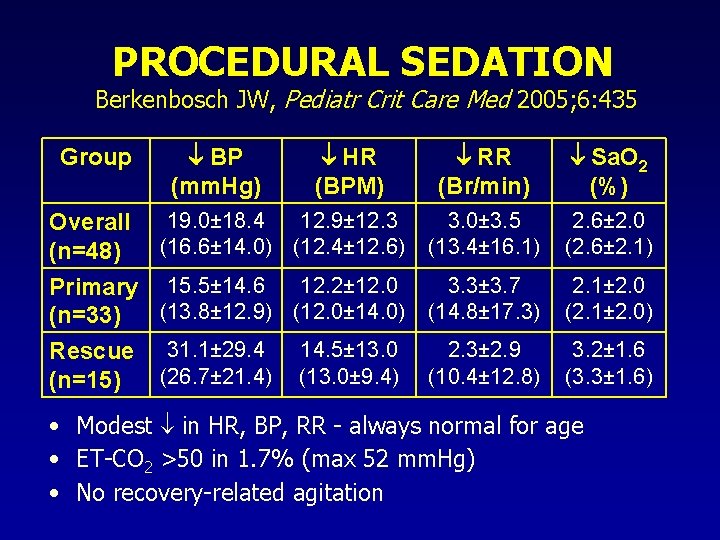
PROCEDURAL SEDATION Berkenbosch JW, Pediatr Crit Care Med 2005; 6: 435 RR (Br/min) Sa. O 2 (%) 3. 0± 3. 5 (13. 4± 16. 1) 2. 6± 2. 0 (2. 6± 2. 1) 3. 3± 3. 7 Primary 15. 5± 14. 6 12. 2± 12. 0 (13. 8± 12. 9) (12. 0± 14. 0) (14. 8± 17. 3) (n=33) 2. 3± 2. 9 Rescue 31. 1± 29. 4 14. 5± 13. 0 (26. 7± 21. 4) (13. 0± 9. 4) (10. 4± 12. 8) (n=15) 2. 1± 2. 0 (2. 1± 2. 0) Group Overall (n=48) BP (mm. Hg) HR (BPM) 19. 0± 18. 4 12. 9± 12. 3 (16. 6± 14. 0) (12. 4± 12. 6) 3. 2± 1. 6 (3. 3± 1. 6) • Modest in HR, BP, RR - always normal for age • ET-CO 2 >50 in 1. 7% (max 52 mm. Hg) • No recovery-related agitation

PROCEDURAL SEDATION • Only 2 comparative trials to date: • Koroglu A, Br J Anaesth 2005; 94: 821 • • Dex vs midazolam for MRI sedation 80 patients, 1 -7 yrs Dex: 1 ug/kg bolus, then 0. 5 ug/kg/hr Midazolam: 0. 2 mg/kg, then 0. 36 mg/kg/hr Efficacy: 32/40 (dex) vs 8/40 (midazolam) Onset: 19 min (dex) vs 35 min (midazolam) Similar CV effects - nothing significant • Concl: dex > efficacy vs midazolam • Problem – midaz rarely sole agent for MRI

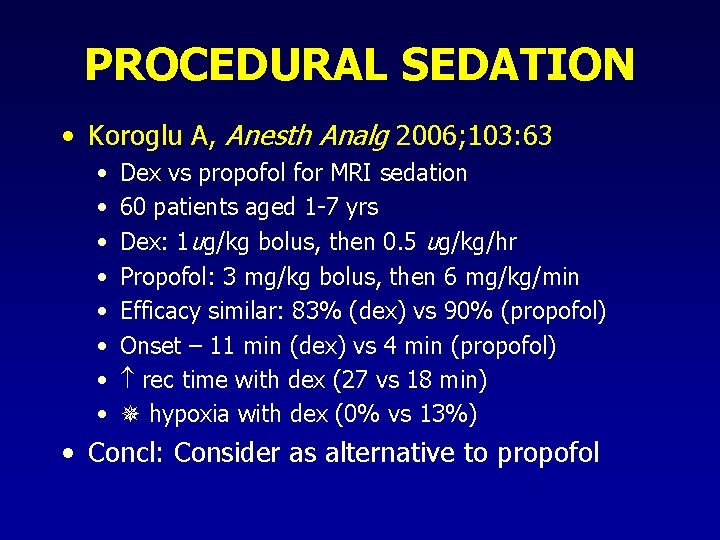
PROCEDURAL SEDATION • Koroglu A, Anesth Analg 2006; 103: 63 • • Dex vs propofol for MRI sedation 60 patients aged 1 -7 yrs Dex: 1 ug/kg bolus, then 0. 5 ug/kg/hr Propofol: 3 mg/kg bolus, then 6 mg/kg/min Efficacy similar: 83% (dex) vs 90% (propofol) Onset – 11 min (dex) vs 4 min (propofol) rec time with dex (27 vs 18 min) hypoxia with dex (0% vs 13%) • Concl: Consider as alternative to propofol

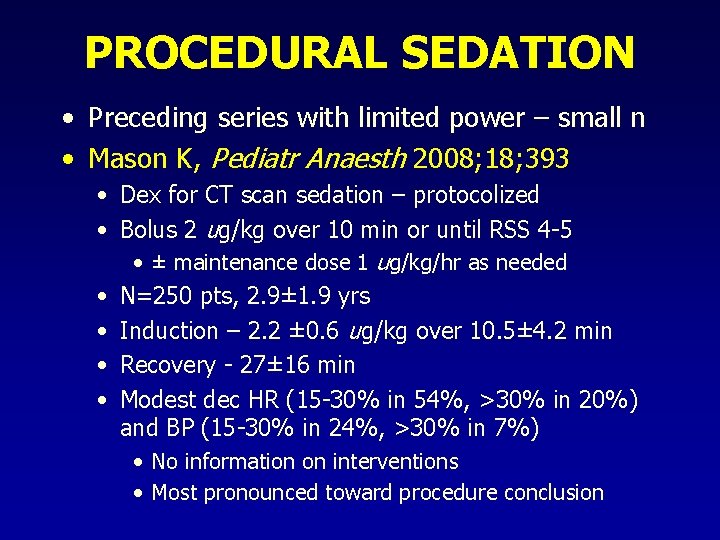
PROCEDURAL SEDATION • Preceding series with limited power – small n • Mason K, Pediatr Anaesth 2008; 18; 393 • Dex for CT scan sedation – protocolized • Bolus 2 ug/kg over 10 min or until RSS 4 -5 • ± maintenance dose 1 ug/kg/hr as needed • N=250 pts, 2. 9± 1. 9 yrs • Induction – 2. 2 ± 0. 6 ug/kg over 10. 5± 4. 2 min • Recovery - 27± 16 min • Modest dec HR (15 -30% in 54%, >30% in 20%) and BP (15 -30% in 24%, >30% in 7%) • No information on interventions • Most pronounced toward procedure conclusion

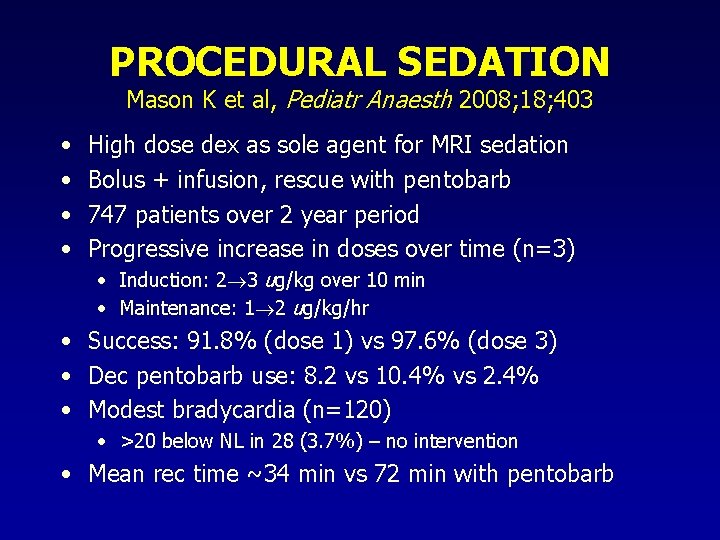
PROCEDURAL SEDATION Mason K et al, Pediatr Anaesth 2008; 18; 403 • • High dose dex as sole agent for MRI sedation Bolus + infusion, rescue with pentobarb 747 patients over 2 year period Progressive increase in doses over time (n=3) • Induction: 2 3 ug/kg over 10 min • Maintenance: 1 2 ug/kg/hr • Success: 91. 8% (dose 1) vs 97. 6% (dose 3) • Dec pentobarb use: 8. 2 vs 10. 4% vs 2. 4% • Modest bradycardia (n=120) • >20 below NL in 28 (3. 7%) – no intervention • Mean rec time ~34 min vs 72 min with pentobarb

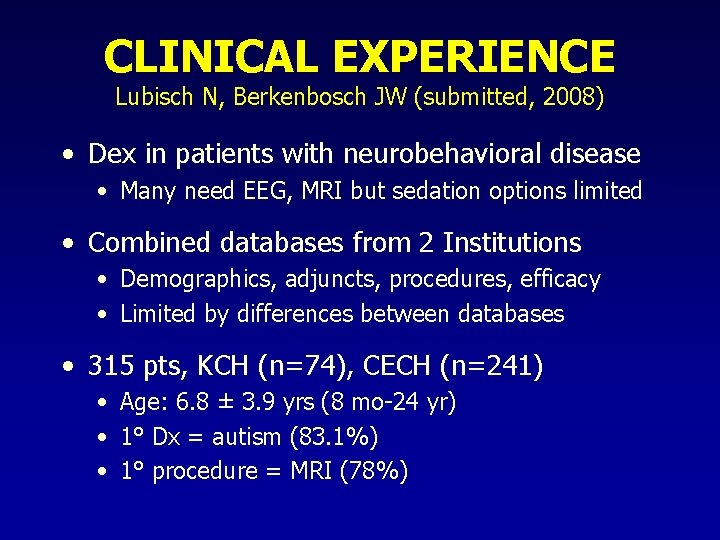
CLINICAL EXPERIENCE Lubisch N, Berkenbosch JW (submitted, 2008) • Dex in patients with neurobehavioral disease • Many need EEG, MRI but sedation options limited • Combined databases from 2 Institutions • Demographics, adjuncts, procedures, efficacy • Limited by differences between databases • 315 pts, KCH (n=74), CECH (n=241) • Age: 6. 8 ± 3. 9 yrs (8 mo-24 yr) • 1° Dx = autism (83. 1%) • 1° procedure = MRI (78%)

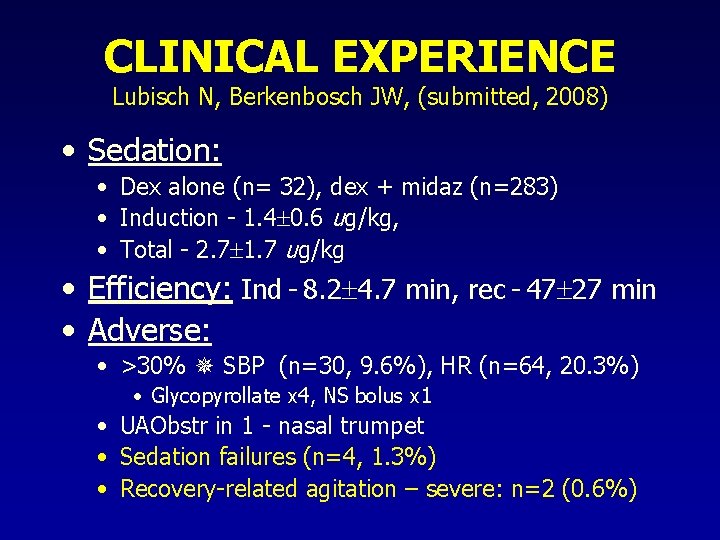
CLINICAL EXPERIENCE Lubisch N, Berkenbosch JW, (submitted, 2008) • Sedation: • Dex alone (n= 32), dex + midaz (n=283) • Induction - 1. 4 0. 6 ug/kg, • Total - 2. 7 1. 7 ug/kg • Efficiency: Ind - 8. 2 4. 7 min, rec - 47 27 min • Adverse: • >30% SBP (n=30, 9. 6%), HR (n=64, 20. 3%) • Glycopyrollate x 4, NS bolus x 1 • UAObstr in 1 - nasal trumpet • Sedation failures (n=4, 1. 3%) • Recovery-related agitation – severe: n=2 (0. 6%)

PSRC EXPERIENCE Berkenbosch JW, Lubisch N, PSRC (in preparation) • Major limitation of single Institution studies is sample size and power. • Pediatric Sedation Research Consortium – 37 institution collaborative • July 1, 2004 – Data collection begun • Through 9/2007 – 90, 000+ sedation entries • Database queried from 7/1/2004 – 9/1/2007 for all sedations using dexmedetomidine

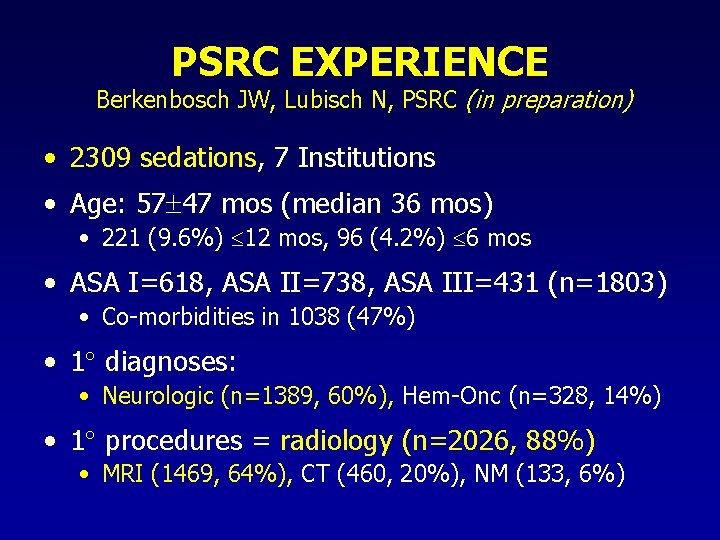
PSRC EXPERIENCE Berkenbosch JW, Lubisch N, PSRC (in preparation) • 2309 sedations, 7 Institutions • Age: 57 47 mos (median 36 mos) • 221 (9. 6%) 12 mos, 96 (4. 2%) 6 mos • ASA I=618, ASA II=738, ASA III=431 (n=1803) • Co-morbidities in 1038 (47%) • 1 diagnoses: • Neurologic (n=1389, 60%), Hem-Onc (n=328, 14%) • 1 procedures = radiology (n=2026, 88%) • MRI (1469, 64%), CT (460, 20%), NM (133, 6%)

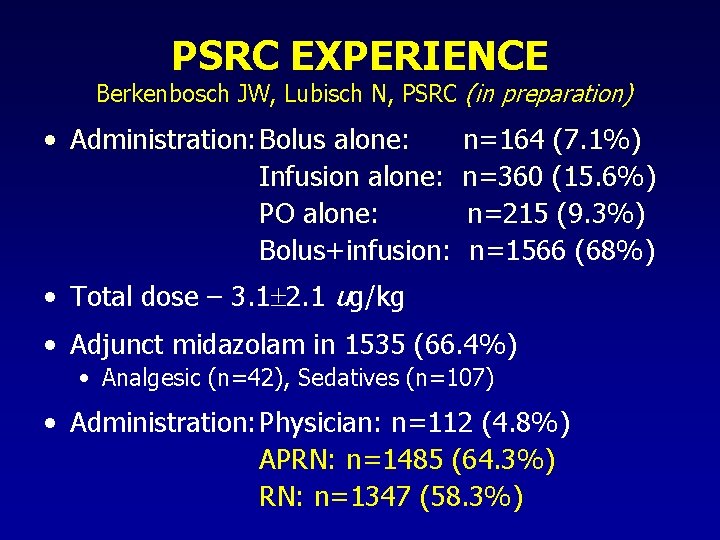
PSRC EXPERIENCE Berkenbosch JW, Lubisch N, PSRC (in preparation) • Administration: Bolus alone: Infusion alone: PO alone: Bolus+infusion: n=164 (7. 1%) n=360 (15. 6%) n=215 (9. 3%) n=1566 (68%) • Total dose – 3. 1 2. 1 ug/kg • Adjunct midazolam in 1535 (66. 4%) • Analgesic (n=42), Sedatives (n=107) • Administration: Physician: n=112 (4. 8%) APRN: n=1485 (64. 3%) RN: n=1347 (58. 3%)

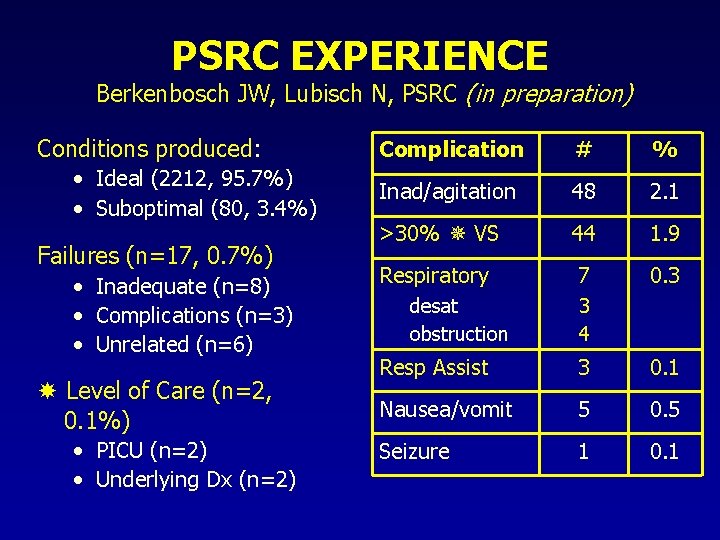
PSRC EXPERIENCE Berkenbosch JW, Lubisch N, PSRC (in preparation) Conditions produced: • Ideal (2212, 95. 7%) • Suboptimal (80, 3. 4%) Failures (n=17, 0. 7%) • Inadequate (n=8) • Complications (n=3) • Unrelated (n=6) Level of Care (n=2, 0. 1%) • PICU (n=2) • Underlying Dx (n=2) Complication # % Inad/agitation 48 2. 1 >30% VS 44 1. 9 Respiratory 7 0. 3 desat obstruction 3 4 Resp Assist 3 0. 1 Nausea/vomit 5 0. 5 Seizure 1 0. 1

PSRC EXPERIENCE Berkenbosch JW, Lubisch N, PSRC (in preparation) • Highly effective • Dex alone – 724/729 (99. 3%) • Dex + Midazolam – 1334/1440 (99. 6%) • Dex + any adjunct – 2298/2309 (99. 5%) • Adverse events favorable compared to PSRC • Respiratory – 1: 329 vs 1: 49 • Airway Intervention – 1: 770 vs 1: 89 • Failed sedation – 1: 210 vs 1: 338 • Availability to/administration by non-physicians

NON-IV USE – ORAL Zub et al, Pediatr Anesth 2005; 932 • Dex (vs of midaz) as premed for OR/IV • Planned IV dex d/t EEG in 9, OR premed in 4 • 7/9 - prior failed attempts with other po • 13 pts, 8. 3± 3 yrs (4 -14) • po dose - 2. 6± 0. 8 ug/kg (1 -4. 2 ug/kg) • Undiluted (100 ug/ml), slowly (buccal >> gastric) • Time to IV placement – 30 -50 min • Success in all, minimal distress • efficacy, efficiency with 3 -4 ug/kg

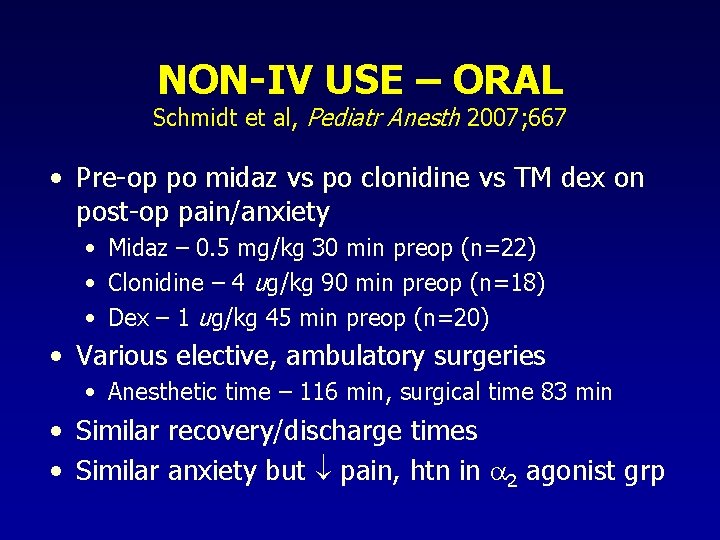
NON-IV USE – ORAL Schmidt et al, Pediatr Anesth 2007; 667 • Pre-op po midaz vs po clonidine vs TM dex on post-op pain/anxiety • Midaz – 0. 5 mg/kg 30 min preop (n=22) • Clonidine – 4 ug/kg 90 min preop (n=18) • Dex – 1 ug/kg 45 min preop (n=20) • Various elective, ambulatory surgeries • Anesthetic time – 116 min, surgical time 83 min • Similar recovery/discharge times • Similar anxiety but pain, htn in 2 agonist grp

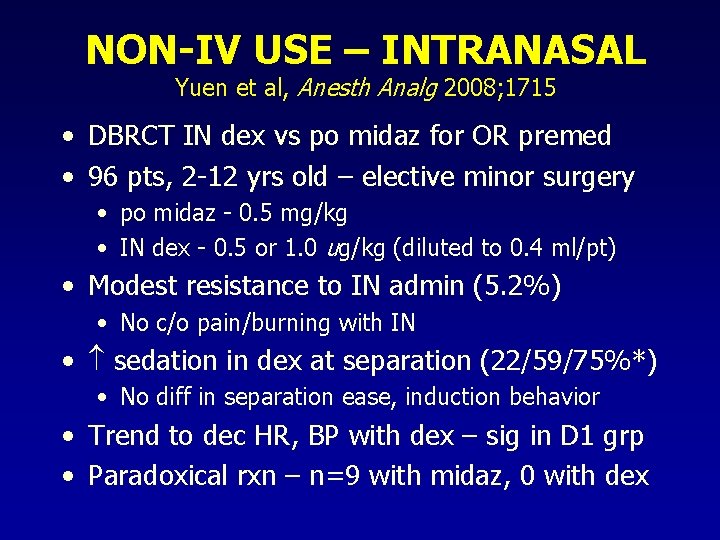
NON-IV USE – INTRANASAL Yuen et al, Anesth Analg 2008; 1715 • DBRCT IN dex vs po midaz for OR premed • 96 pts, 2 -12 yrs old – elective minor surgery • po midaz - 0. 5 mg/kg • IN dex - 0. 5 or 1. 0 ug/kg (diluted to 0. 4 ml/pt) • Modest resistance to IN admin (5. 2%) • No c/o pain/burning with IN • sedation in dex at separation (22/59/75%*) • No diff in separation ease, induction behavior • Trend to dec HR, BP with dex – sig in D 1 grp • Paradoxical rxn – n=9 with midaz, 0 with dex

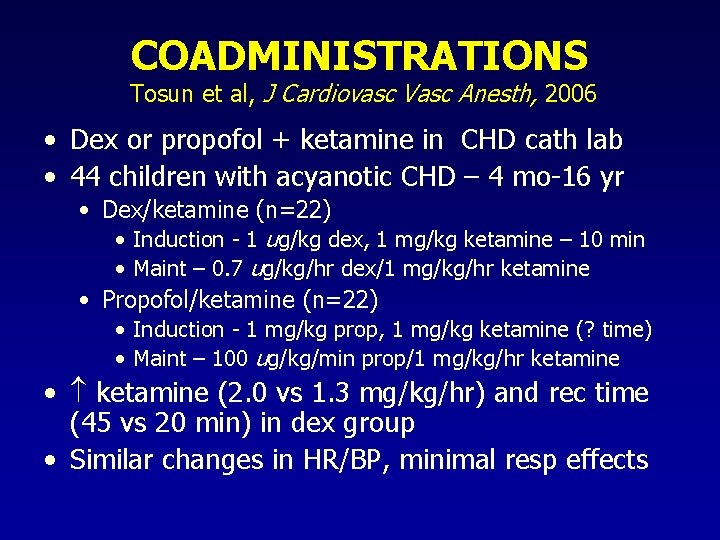
COADMINISTRATIONS Tosun et al, J Cardiovasc Vasc Anesth, 2006 • Dex or propofol + ketamine in CHD cath lab • 44 children with acyanotic CHD – 4 mo-16 yr • Dex/ketamine (n=22) • Induction - 1 ug/kg dex, 1 mg/kg ketamine – 10 min • Maint – 0. 7 ug/kg/hr dex/1 mg/kg/hr ketamine • Propofol/ketamine (n=22) • Induction - 1 mg/kg prop, 1 mg/kg ketamine (? time) • Maint – 100 ug/kg/min prop/1 mg/kg/hr ketamine • ketamine (2. 0 vs 1. 3 mg/kg/hr) and rec time (45 vs 20 min) in dex group • Similar changes in HR/BP, minimal resp effects

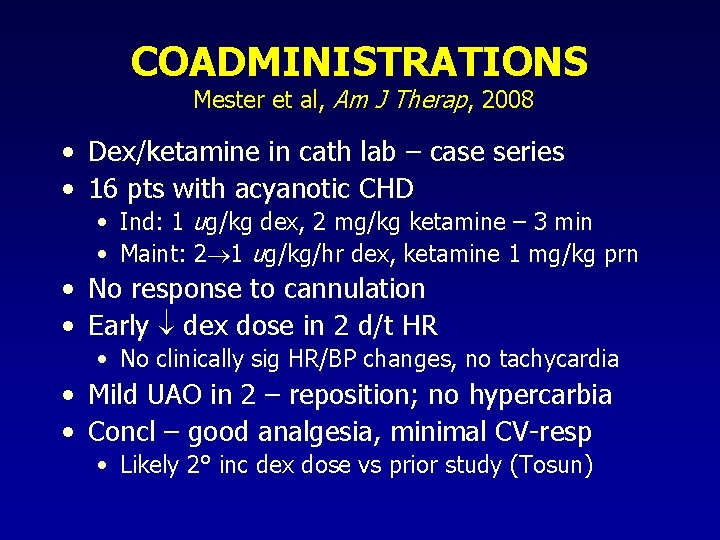
COADMINISTRATIONS Mester et al, Am J Therap, 2008 • Dex/ketamine in cath lab – case series • 16 pts with acyanotic CHD • Ind: 1 ug/kg dex, 2 mg/kg ketamine – 3 min • Maint: 2 1 ug/kg/hr dex, ketamine 1 mg/kg prn • No response to cannulation • Early dex dose in 2 d/t HR • No clinically sig HR/BP changes, no tachycardia • Mild UAO in 2 – reposition; no hypercarbia • Concl – good analgesia, minimal CV-resp • Likely 2° inc dex dose vs prior study (Tosun)

CONCLUSIONS • Effective for non-invasive procedures • Coadmin with analgesics for invasive? ? • Dose moderately higher than for ICU sedation • 2 -3 ug/kg/hr well tolerated medium-term • Lack of recovery-related agitation significant • Minimal compared to chloral, barbiturates • Role of adjunct benzodiazepines unclear • Similar CV, resp vs propofol • availability vs propofol in many venues • Ongoing paucity of comparative reports/trials

PRACTICAL POINTS • IV use: • Dilute to 4 ug/ml in 0. 9% saline • Infusion usually req for lengthy procedures • Use pump for induction bolus – 12 ug/kg/hr = 1 ug/kg over 5 min • Coadmin with midazolam • Appears to induction time, ? rec time • Buccal/transmucosal • Use undiluted (100 ug/ml) drug • Slow drip into oral cavity efficacy, efficiency by swallowing and, therefore, gastric absorption
 Rass
Rass Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals Sédation
Sédation Richmond agitation sedation scale
Richmond agitation sedation scale Padis 2018 guidelines
Padis 2018 guidelines Over-sedation
Over-sedation Rikers sedation scale
Rikers sedation scale Poss scale
Poss scale Pediatric dysphagia resource guide
Pediatric dysphagia resource guide Examples of generic goals and product-specific goals
Examples of generic goals and product-specific goals General goals and specific goals
General goals and specific goals Procedural abstraction example
Procedural abstraction example What is procedural programming
What is procedural programming Dpgt samples
Dpgt samples Classroom dimensions
Classroom dimensions Features of a procedural text
Features of a procedural text Java scripting
Java scripting Procedural abstraction
Procedural abstraction Superskalar
Superskalar Compare procedural semantics and declarative semantics.
Compare procedural semantics and declarative semantics. Substantive vs procedural due process
Substantive vs procedural due process What is procedural fluency
What is procedural fluency 2d procedural animation
2d procedural animation Isa 88
Isa 88 Procedural vs functional
Procedural vs functional Structural vs dataflow verilog
Structural vs dataflow verilog Procedural memory ap psychology
Procedural memory ap psychology Procedural unconscionability
Procedural unconscionability Computable predicates in ai
Computable predicates in ai Wiki
Wiki Due process can be divided into two distinct categories
Due process can be divided into two distinct categories Distributive justice definition
Distributive justice definition Blender procedural generation
Blender procedural generation Procedural model of problem solving
Procedural model of problem solving Procedural abstraction c++
Procedural abstraction c++ Antecedent procedural questions
Antecedent procedural questions Procedural sound generation
Procedural sound generation Equal protection clause
Equal protection clause Procedural self
Procedural self Procedural planning
Procedural planning Procedural plants
Procedural plants Procedural hukommelse
Procedural hukommelse Communicational cohesion
Communicational cohesion