Antiviral Drugs Rennolds Ostrom Ph D rostromchapman edu












![Cobicistat [Tybost] Inhibits liver enzymes that metabolize other antiviral drugs – Potent inhibitor of Cobicistat [Tybost] Inhibits liver enzymes that metabolize other antiviral drugs – Potent inhibitor of](https://slidetodoc.com/presentation_image_h2/a980f64860d6c103ee7221bbb79308bf/image-55.jpg)







- Slides: 77

Antiviral Drugs Rennolds Ostrom, Ph. D. rostrom@chapman. edu

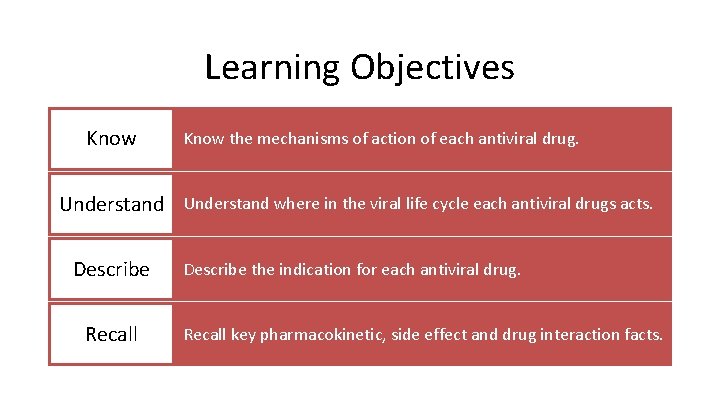
Learning Objectives Know the mechanisms of action of each antiviral drug. Understand where in the viral life cycle each antiviral drugs acts. Describe Recall Describe the indication for each antiviral drug. Recall key pharmacokinetic, side effect and drug interaction facts.

All antivirals are virustatic Drugs don’t kill viruses! Theoretically, antiviral drugs can be targeted at: • Viral attachment to cell outer membranes • Host cell membrane receptors that may be involved in viral entry • Shedding of the protein coat • Virus-specific DNA polymerase or DNAdependent RNA polymerase • Translation of viral m. RNA which differs from host cell m. RNA • Specific virus-coded enzymes that are essential for viral replication and assembly We will cover the antiviral drugs in order of these life cycle steps

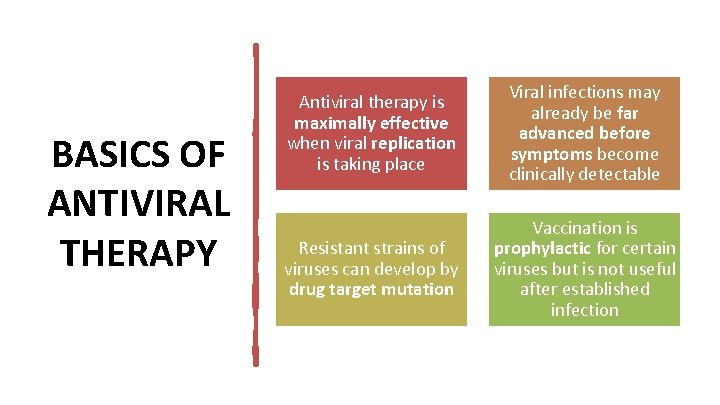
BASICS OF ANTIVIRAL THERAPY Antiviral therapy is maximally effective when viral replication is taking place Viral infections may already be far advanced before symptoms become clinically detectable Resistant strains of viruses can develop by drug target mutation Vaccination is prophylactic for certain viruses but is not useful after established infection

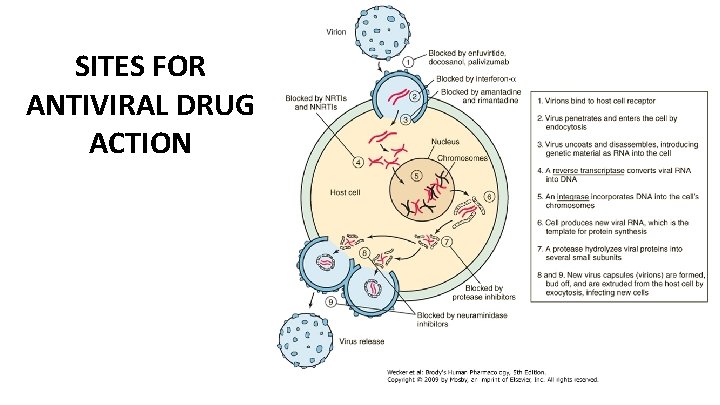
SITES FOR ANTIVIRAL DRUG ACTION

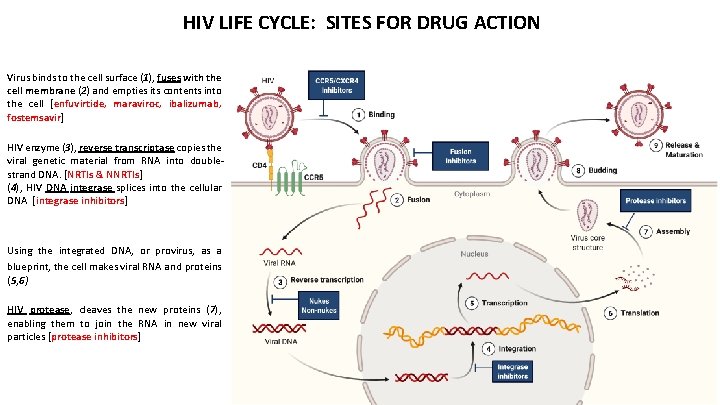
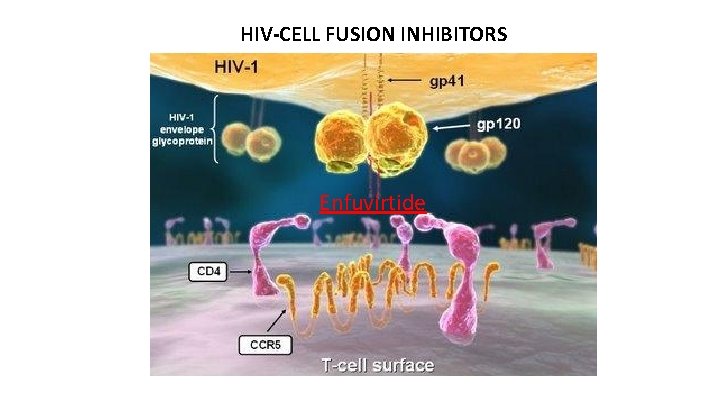
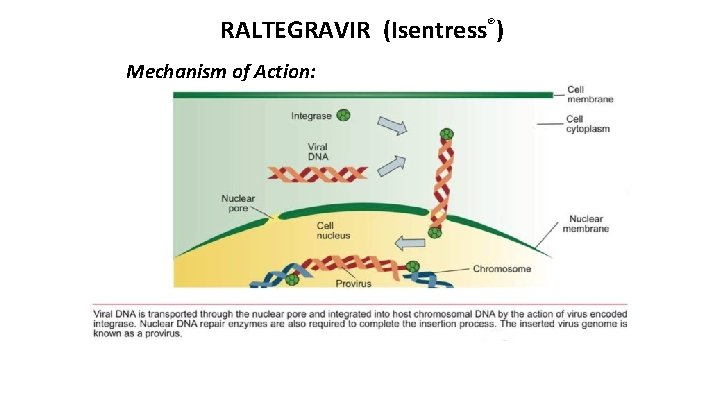
HIV LIFE CYCLE: SITES FOR DRUG ACTION Virus binds to the cell surface (1), fuses with the cell membrane (2) and empties its contents into the cell [enfuvirtide, maraviroc, ibalizumab, fostemsavir] HIV enzyme (3), reverse transcriptase copies the viral genetic material from RNA into doublestrand DNA. [NRTIs & NNRTIs] (4), HIV DNA integrase splices into the cellular DNA [integrase inhibitors] Using the integrated DNA, or provirus, as a blueprint, the cell makes viral RNA and proteins (5, 6) HIV protease, cleaves the new proteins (7), enabling them to join the RNA in new viral particles [protease inhibitors]

INHIBITORS OF CELL PENETRATION HIV ENTRY

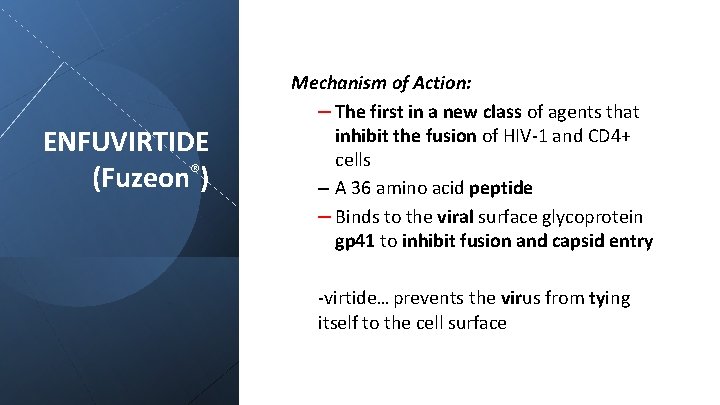
ENFUVIRTIDE (Fuzeon®) Mechanism of Action: – The first in a new class of agents that inhibit the fusion of HIV-1 and CD 4+ cells – A 36 amino acid peptide – Binds to the viral surface glycoprotein gp 41 to inhibit fusion and capsid entry -virtide… prevents the virus from tying itself to the cell surface

HIV-CELL FUSION INHIBITORS Enfuvirtide

Pharmacokinetics: 90 mg (1 m. L) bid, s. c. injection (upper arm, anterior thigh, or abdomen) Plasma t 1/2 of ~4 hours Undergoes catabolism into component amino acids ENFUVIRTIDE (Fuzeon®) Clinical: A polypeptide for use in combination with other antiretroviral agents for the treatment of HIV-1 infection Resistance observed by mutation of gp 41 glycoprotein Pharmacovigilance: Pain (“wasp sting”) and erythema at local injection site Diarrhea, nausea and fatigue No effect on cyp 450 enzymes Annual patient cost for Fuzeon: >$20, 000!!

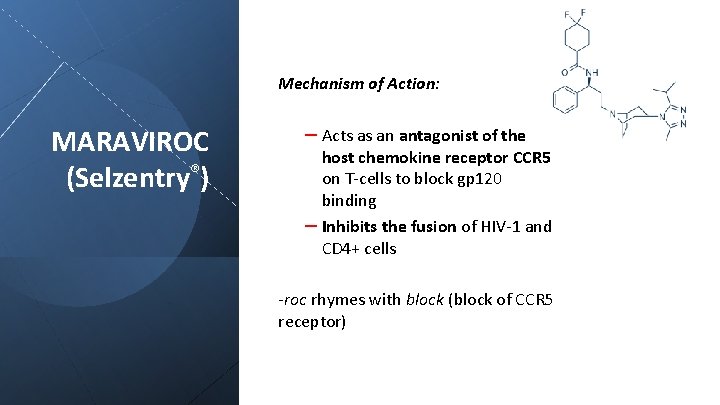
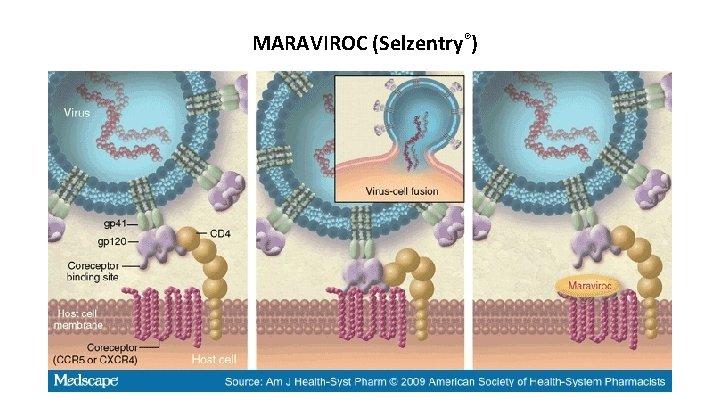
Mechanism of Action: MARAVIROC (Selzentry®) – Acts as an antagonist of the host chemokine receptor CCR 5 on T-cells to block gp 120 binding – Inhibits the fusion of HIV-1 and CD 4+ cells -roc rhymes with block (block of CCR 5 receptor)

MARAVIROC (Selzentry®)

MARAVIROC (Selzentry®) Pharmacokinetics: 150 mg p. o. twice daily Plasma t 1/2 of 14 -18 hours Liver metabolism via CYP 3 A + renal clearance of 25% of drug Clinical: CCR 5 Chemokine receptor antagonist for use in combination with other antiretroviral agents for HIV-1 infection An HIV tropism test should be done to confirm CCR 5 tropism (HIV can enter via either CCR 5 or CXCR 4 receptors) Pharmacovigilance: Cough, fever, upper respiratory infections, rash, musculoskeletal symptoms, abdominal pain, and dizziness Increased incidence of myocardial ischemia and/or infarction Exacerbation of preexisting liver dysfunction, for example in those co-infected with viral hepatitis B or C Resistance observed by mutation of the HIV gp 120 glycoprotein

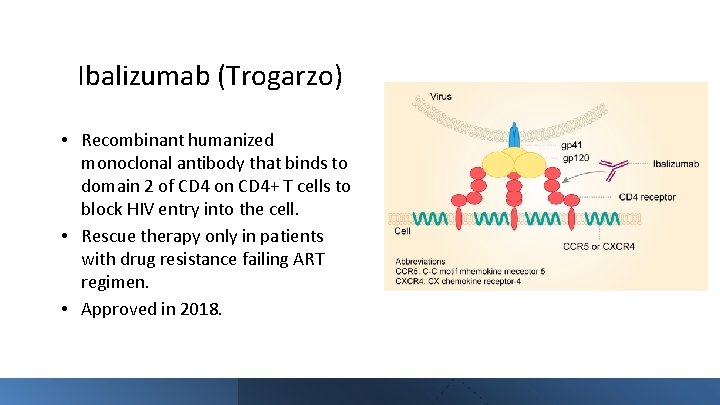
Ibalizumab (Trogarzo) • Recombinant humanized monoclonal antibody that binds to domain 2 of CD 4 on CD 4+ T cells to block HIV entry into the cell. • Rescue therapy only in patients with drug resistance failing ART regimen. • Approved in 2018.

Ibalizumab (Trogarzo) • Clinical: – 82. 5% of patients achieved more than ~3 -fold reduction (0. 5 log reduction) in vial load 7 days after loading dose – After 24 weeks 48% of patients had • Common AE: – Diarrhea, dizziness, nausea and rash • Severe AE: – Rash and changes in the immune system (immune reconstitution syndrome)

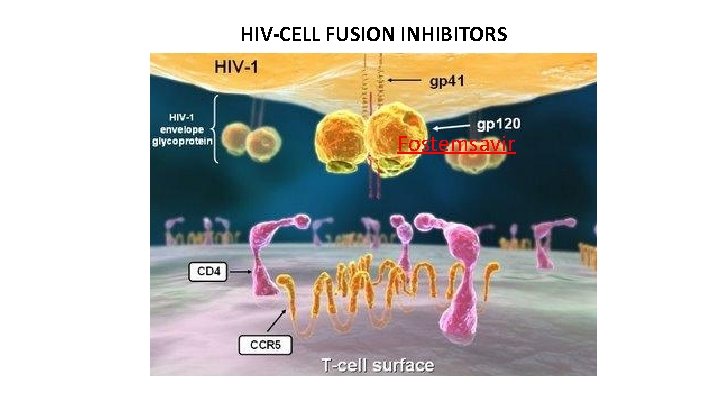
Fostemsavir (Rukobia®) Mechanism of Action: – Binds to HIV envelope glycoprotein 120 (gp 120) prevents the gp 120 conformational change required for attachment to the host CD 4 cell surface receptor – Prodrug that is hydrolyzed to the active drug temsavir Approved July 2020

HIV-CELL FUSION INHIBITORS Fostemsavir

INHIBITORS OF VIRAL DNA & RNA SYNTHESIS

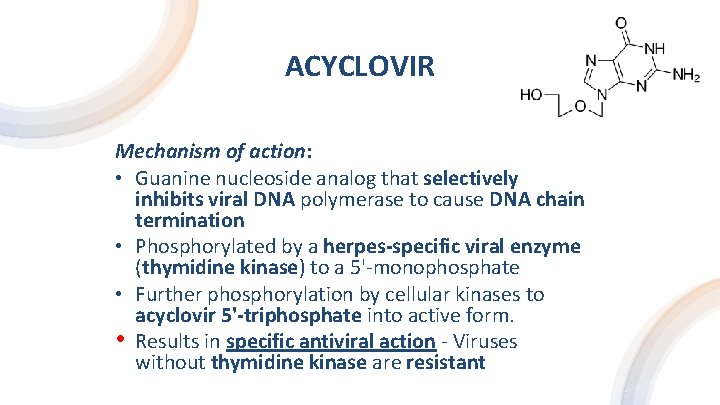
ACYCLOVIR Mechanism of action: • Guanine nucleoside analog that selectively inhibits viral DNA polymerase to cause DNA chain termination • Phosphorylated by a herpes-specific viral enzyme (thymidine kinase) to a 5'-monophosphate • Further phosphorylation by cellular kinases to acyclovir 5'-triphosphate into active form. • Results in specific antiviral action - Viruses without thymidine kinase are resistant

ACYCLOVIR Pharmacokinetics: Sodium salt form in capsules (200 mg), ointment (5%) in PEG and powder for reconstitution for i. v. use Elimination t 1/2 of 2. 5 hours, excreted mostly unchanged in the urine with <10% being oxidized 15 -30% bioavailability, penetrates the CNS ( 50% of plasma levels) Valacyclovir, the valyl ester prodrug of acyclovir, has 3 -5 X greater bioavailability Clinical: High specificity against herpes simplex 1 Topically reduces viral shedding and improves rate of healing in primary genital herpes Orally effective against both primary & recurrent genital herpes Used prophylactically when patients are immunologically compromised Pharmacovigilance: Low toxicity with oral use, occasional headaches, nausea or amnesia High alkalinity (p. H 10 -11) of sodium salt can cause local phlebitis due to extravasation at i. v. infusion site Vomiting and hypotension along with CNS effects Crystallizes in renal tubules unless the i. v. rate is slow and the patient is well hydrated Highest therapeutic index of nucleoside antivirals

GANCICLOVIR Mechanism of Action A guanine nucleoside analog similar to acyclovir Selective uptake by infected cells • Phosphorylated by viral thymidine kinase and host cellular kinases to the triphosphate • The triphosphate inhibits d. GTP incorporation into DNA and selectively inhibits viral DNA polymerase • Incorporation in viral DNA limits viral DNA chain elongation

GANCICLOVIR Pharmacokinetics i. v. use, no oral bioavailability 3 -4 hr half-life Eliminated unchanged in urine Clinical Active in vitro against all herpes viruses Toxicity limits use to life-threatening or sight-threatening cytomegalovirus (CMV) Main clinical use is the treatment of CMV in immunocompromised patients as an i. v. drug Pharmacovigilance Reversible bone marrow suppression with neutropenia and thrombocytopenia 5 -15% of patients have CNS effects, including behavioral changes; psychosis; convulsions and coma Ganciclovir + imipenem will cause seizures ~2% have anemia, rash, fever or nausea

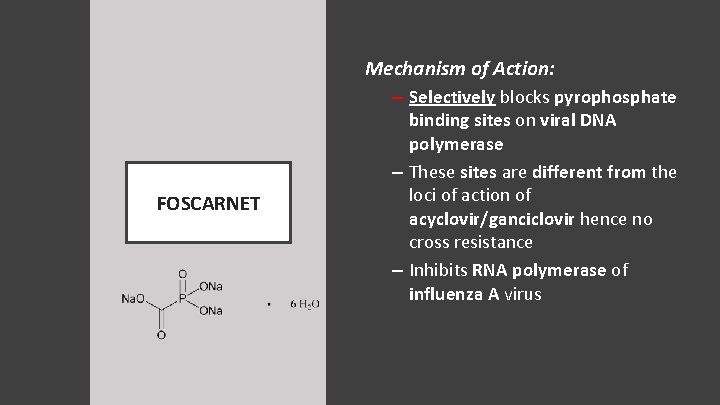
Mechanism of Action: – Selectively blocks pyrophosphate FOSCARNET binding sites on viral DNA polymerase – These sites are different from the loci of action of acyclovir/ganciclovir hence no cross resistance – Inhibits RNA polymerase of influenza A virus

Pharmacokinetics: Short half-life of 0. 7 -3. 5 hours after i. v. infusion and no metabolic conversion Predominant (88%) renal excretion with small amounts accumulating in bone Penetrates the CNS and macrophages FOSCARNET Clinical: Effective parenteral against herpes virus including CMV Main clinical use is to treat CMV retinitis in AIDS patients Active against strains resistant to acyclovir and to ganciclovir because of different sites of action Resistance occurs due to mutation of the pyro-phosphate binding site Pharmacovigilance: Most common adverse effect is diminished renal function with prolonged administration Increases fluid intake and urine output (i. e. , drug-induced diabetes insipidus) Associated with malaise, nausea vomiting, fatigue, headaches and anemia without granulocytopenia

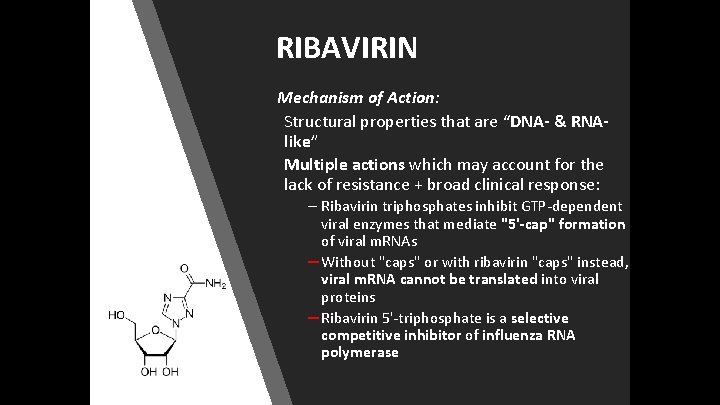
RIBAVIRIN Mechanism of Action: Structural properties that are “DNA- & RNAlike” Multiple actions which may account for the lack of resistance + broad clinical response: – Ribavirin triphosphates inhibit GTP-dependent viral enzymes that mediate "5'-cap" formation of viral m. RNAs – Without "caps" or with ribavirin "caps" instead, viral m. RNA cannot be translated into viral proteins – Ribavirin 5'-triphosphate is a selective competitive inhibitor of influenza RNA polymerase

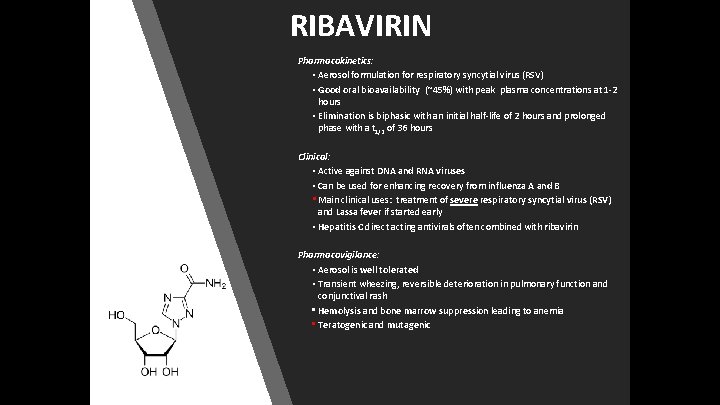
RIBAVIRIN Pharmacokinetics: • Aerosol formulation for respiratory syncytial virus (RSV) • Good oral bioavailability (~45%) with peak plasma concentrations at 1 -2 hours • Elimination is biphasic with an initial half-life of 2 hours and prolonged phase with a t 1/2 of 36 hours Clinical: • Active against DNA and RNA viruses • Can be used for enhancing recovery from influenza A and B • Main clinical uses: treatment of severe respiratory syncytial virus (RSV) and Lassa fever if started early • Hepatitis C direct acting antivirals often combined with ribavirin Pharmacovigilance: • Aerosol is well tolerated • Transient wheezing, reversible deterioration in pulmonary function and conjunctival rash • Hemolysis and bone marrow suppression leading to anemia • Teratogenic and mutagenic

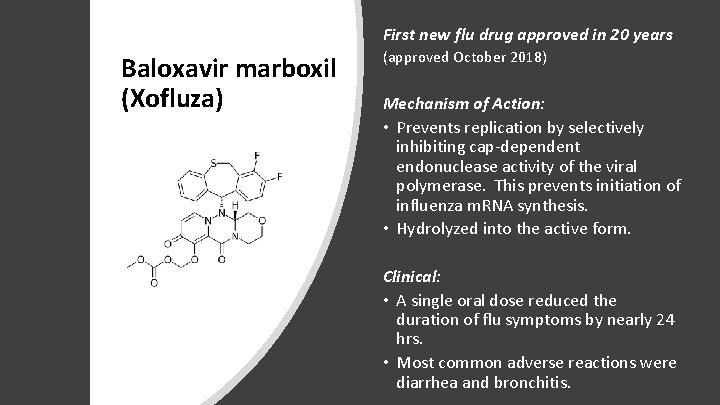
First new flu drug approved in 20 years Baloxavir marboxil (Xofluza) (approved October 2018) Mechanism of Action: • Prevents replication by selectively inhibiting cap-dependent endonuclease activity of the viral polymerase. This prevents initiation of influenza m. RNA synthesis. • Hydrolyzed into the active form. Clinical: • A single oral dose reduced the duration of flu symptoms by nearly 24 hrs. • Most common adverse reactions were diarrhea and bronchitis.

NUCLEOSIDE/NUCLEOTIDE HIV REVERSE TRANSCRIPTASE INHIBITORS “NUKES”

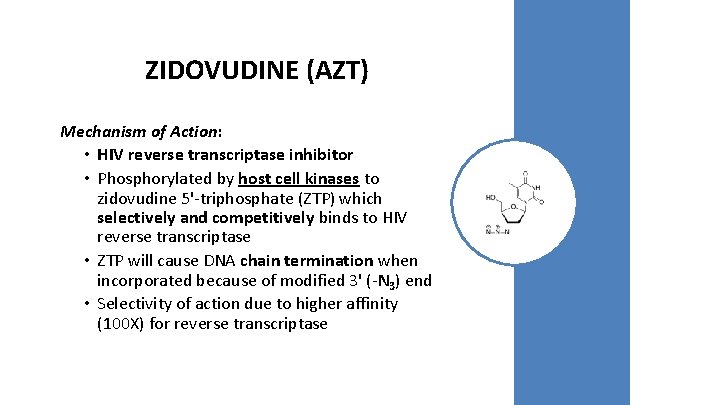
ZIDOVUDINE (AZT) Mechanism of Action: • HIV reverse transcriptase inhibitor • Phosphorylated by host cell kinases to zidovudine 5'-triphosphate (ZTP) which selectively and competitively binds to HIV reverse transcriptase • ZTP will cause DNA chain termination when incorporated because of modified 3' (-N 3) end • Selectivity of action due to higher affinity (100 X) for reverse transcriptase

ZIDOVUDINE (AZT) Pharmacokinetics: – Oral absorption is rapid with bioavailability of 60 -65% – Half-life is short (60 min) – Penetrates the CNS – Metabolized (85%) to 5'-glucuronide and excreted predominantly in urine – Drugs inhibiting glucuronyl transferase (e. g. , aspirin, indomethacin) will increase risk of hematological toxicity Clinical: – Deoxythymidine analog originally developed for cancer – Older (1987) treatment for AIDS, active against HIV-1 in combination with other antiretroviral agents – Mainly used to reduce vertical transmission during pregnancy Pharmacovigilance: – Most commonly (< 45%), granulocytopenia and anemia both of which are dose limiting – In addition: headaches, nausea, insomnia, agitation and seizures

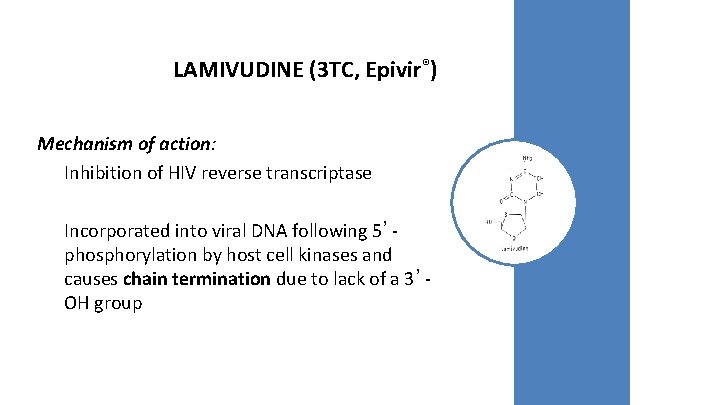
LAMIVUDINE (3 TC, Epivir®) Mechanism of action: Inhibition of HIV reverse transcriptase Incorporated into viral DNA following 5’phosphorylation by host cell kinases and causes chain termination due to lack of a 3’OH group

LAMIVUDINE (3 TC, Epivir®) Pharmacokinetics: – Penetrates CNS after oral administration – t½ of 5 -7 hrs – Excreted unchanged in urine Clinical: – HIV infection in patients refractory to or intolerant of other available anti-retroviral agents – Use in combination only ® – Combined with zidovudine (Combivir ) or zidovudine + abacavir ® (Trizivir ) results in synergistic inhibition of HIV – Used for the treatment of hepatitis B Pharmacovigilance: – Similar to those of zidovudine but with much lower frequency – G. I. disturbances and neutropenia – Black box warning for lactic acidosis and severe hepatomegaly (do not combine with stavudine)

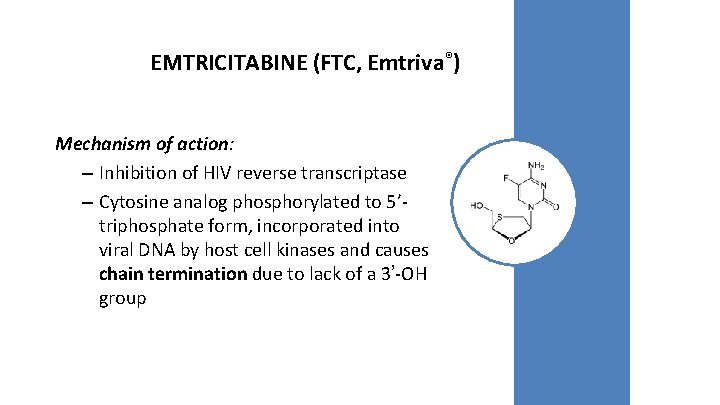
EMTRICITABINE (FTC, Emtriva®) Mechanism of action: – Inhibition of HIV reverse transcriptase – Cytosine analog phosphorylated to 5’triphosphate form, incorporated into viral DNA by host cell kinases and causes chain termination due to lack of a 3’-OH group

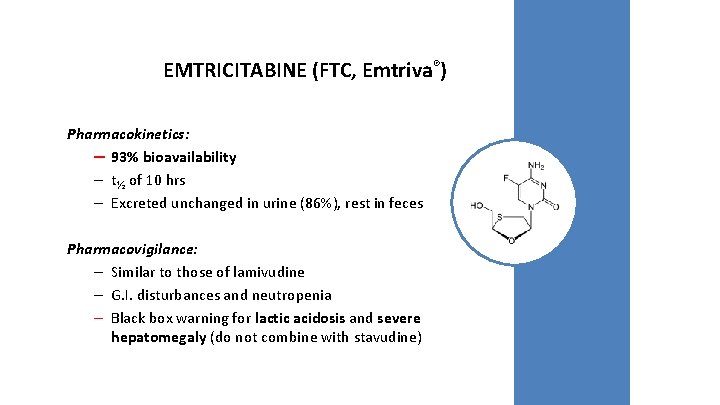
EMTRICITABINE (FTC, Emtriva®) Pharmacokinetics: – 93% bioavailability – t½ of 10 hrs – Excreted unchanged in urine (86%), rest in feces Pharmacovigilance: – Similar to those of lamivudine – G. I. disturbances and neutropenia – Black box warning for lactic acidosis and severe hepatomegaly (do not combine with stavudine)

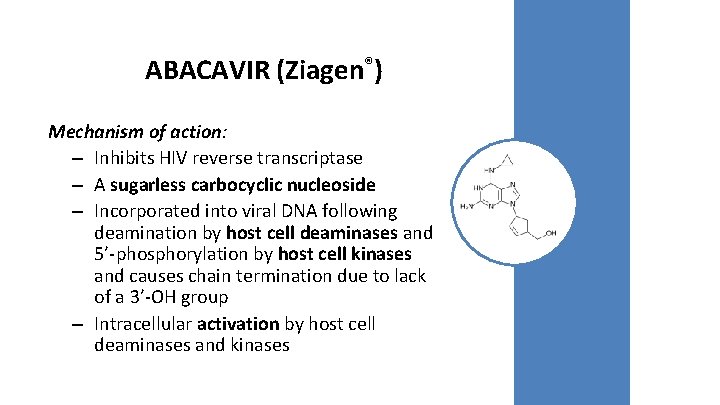
ABACAVIR (Ziagen®) Mechanism of action: – Inhibits HIV reverse transcriptase – A sugarless carbocyclic nucleoside – Incorporated into viral DNA following deamination by host cell deaminases and 5’-phosphorylation by host cell kinases and causes chain termination due to lack of a 3’-OH group – Intracellular activation by host cell deaminases and kinases

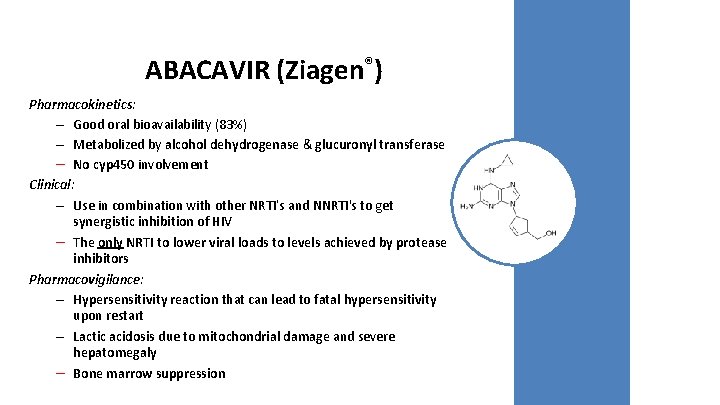
ABACAVIR (Ziagen®) Pharmacokinetics: – Good oral bioavailability (83%) – Metabolized by alcohol dehydrogenase & glucuronyl transferase – No cyp 450 involvement Clinical: – Use in combination with other NRTI’s and NNRTI's to get synergistic inhibition of HIV – The only NRTI to lower viral loads to levels achieved by protease inhibitors Pharmacovigilance: – Hypersensitivity reaction that can lead to fatal hypersensitivity upon restart – Lactic acidosis due to mitochondrial damage and severe hepatomegaly – Bone marrow suppression

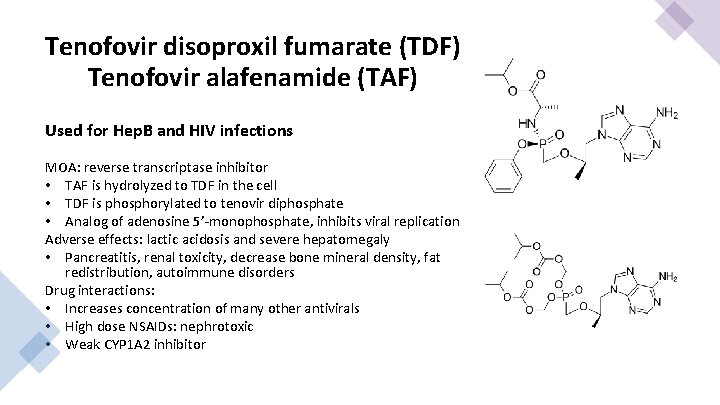
Tenofovir disoproxil fumarate (TDF) Tenofovir alafenamide (TAF) Used for Hep. B and HIV infections MOA: reverse transcriptase inhibitor • TAF is hydrolyzed to TDF in the cell • TDF is phosphorylated to tenovir diphosphate • Analog of adenosine 5’-monophosphate, inhibits viral replication Adverse effects: lactic acidosis and severe hepatomegaly • Pancreatitis, renal toxicity, decrease bone mineral density, fat redistribution, autoimmune disorders Drug interactions: • Increases concentration of many other antivirals • High dose NSAIDs: nephrotoxic • Weak CYP 1 A 2 inhibitor

NONNUCLEOSIDE/NUCLEOTIDE HIV REVERSE TRANSCRIPTASE INHIBITORS “NON-NUKES”

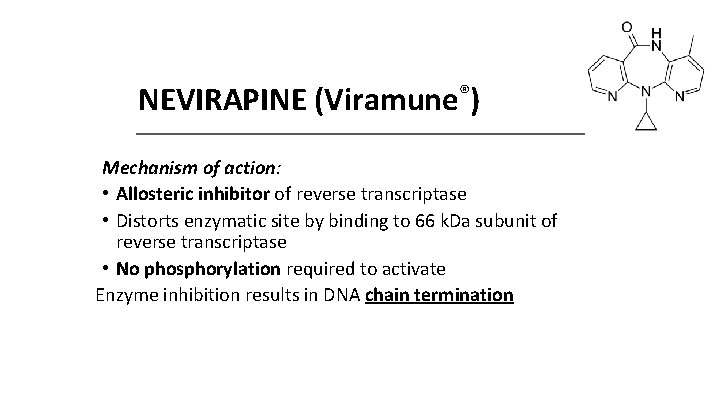
NEVIRAPINE (Viramune®) Mechanism of action: • Allosteric inhibitor of reverse transcriptase • Distorts enzymatic site by binding to 66 k. Da subunit of reverse transcriptase • No phosphorylation required to activate Enzyme inhibition results in DNA chain termination

NEVIRAPINE (Viramune®) Pharmacokinetics: • Metabolized by CYP 3 A 4 and self-induces • Extensive liver metabolism and renal excretion • Half life 25 -30 hrs with 98% oral bioavailability • Penetrates CNS Clinical: • Used for HIV infection in patients refractory to nucleoside analogs • Alternative to efavirenz as initial non-nuke component of HAART • Use in combination with other antiretroviral agents Pharmacovigilance: • Common side-effects are rash and liver dysfunction • Black box warning for hepatoxicity, liver function should be assessed before and during therapy • Discontinue if severe (i. e. , fever, blistering, muscle and joint aches) rash occurs

EFAVIRENZ (Sustiva®) Mechanism of action: • A benoxazinone non-nucleoside reverse transcriptase inhibitor (NNRTI) • Allosterically inhibits reverse transcriptase

EFAVIRENZ (Sustiva®) Pharmacokinetics: • Good oral bioavailability • Prolonged half life of 52 -76 hours allows once a day dosing • Liver metabolism by CYP 3 A 4 and causes CYP 3 A 4 induction • Limited CNS penetration Clinical: • HIV-1 infection in combination with other antiretroviral agents • Recommended as one of the NNRTI’s to be used in initial HAART • Resistance and cross resistance to other NNRTI’s observed Pharmacovigilance: • CNS symptoms including delusions and inappropriate behavior • Dizziness, impaired concentration, somnolence and insomnia • Skin rash, hyperlipidemia and gynecomastia • Drug interactions with drugs that induce or are metabolized by CYP 3 A 4

Doravirine (Pifeltro®) Available alone (100 mg) or in combination with lamivudine+TDF (Delstrigo®) • Approved for HIV-1 infection in combination with other antiretroviral agents • FDA approved on 8/31/2018 • Combination therapy showed equivalent viral suppression and lower adverse effects and discontinuation rate than efavirenz/emtricitabine/TDF combination. • T 1/2: 15 hr • Metabolized by CYP 3 A 4 • Too early to know anything about resistance and cross resistance but likely similar to other agents in this class.

HIV PROTEASE INHIBITORS

LOPINAVIR/RITONAVIR (Kaletra®) Mechanism of action: – Lopinavir is a selective HIV protease inhibitor – Ritonavir inhibits CYP 3 A and blocks the liver's ability to destroy lopinavir

LOPINAVIR/RITONAVIR (Kaletra®) Pharmacokinetics: – Plasma t 1/2 of 5 -6 hours (lopinavir) – Liver metabolism by CYP 3 A subfamily and CYP 2 D 6 into glucuronidated conjugates of oxidized metabolites Clinical: – A 4: 1 ratio of lopinavir: ritonavir – Treatment HIV-1 infection in combination with other antiretroviral agents in salvage therapy – Effective in individuals who are resistant to other PIs – Resistance and cross resistance to other protease inhibitors observed in vitro Pharmacovigilance: – Gastrointestinal disturbances (e. g. , nausea, diarrhea) – Fatigue & headache – Pancreatitis (do not combine with didanosine) – Redistribution and accumulation of body fat – Inhibits CYP 3 A 4 and will interact with drugs that are metabolized by CYP 3 A 4

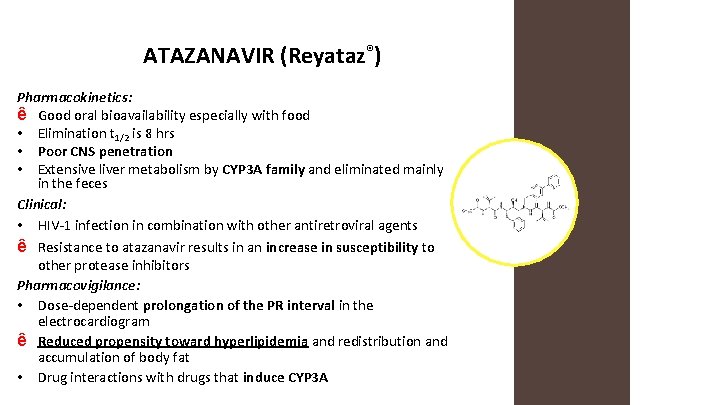
ATAZANAVIR (Reyataz®) Mechanism of Action: • An azapeptide HIV-1 protease inhibitor

ATAZANAVIR (Reyataz®) Pharmacokinetics: ê Good oral bioavailability especially with food • Elimination t 1/2 is 8 hrs • Poor CNS penetration • Extensive liver metabolism by CYP 3 A family and eliminated mainly in the feces Clinical: • HIV-1 infection in combination with other antiretroviral agents ê Resistance to atazanavir results in an increase in susceptibility to other protease inhibitors Pharmacovigilance: • Dose-dependent prolongation of the PR interval in the electrocardiogram ê Reduced propensity toward hyperlipidemia and redistribution and accumulation of body fat • Drug interactions with drugs that induce CYP 3 A

Darunavir • CYP 3 A 4 metabolized Other HIV Protease Inhibitors Fosamprenavir • Prodrug rapidly converted to amprenavir by cellular phosphatases Nelfinavir • Rarely used anymore due to ADRs

HIV INTEGRASE INHIBITORS in. TEGrase

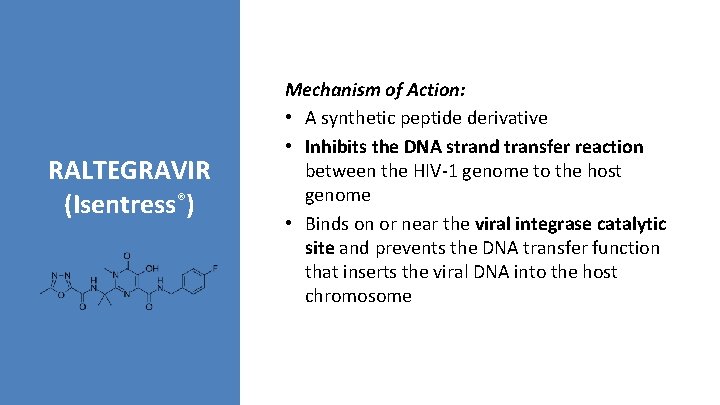
RALTEGRAVIR (Isentress®) Mechanism of Action: • A synthetic peptide derivative • Inhibits the DNA strand transfer reaction between the HIV-1 genome to the host genome • Binds on or near the viral integrase catalytic site and prevents the DNA transfer function that inserts the viral DNA into the host chromosome

RALTEGRAVIR (Isentress®) Mechanism of Action:

RALTEGRAVIR (Isentress®) Pharmacokinetics: • Adult recommended dosage of 400 mg p. o. b. i. d. (~$9900/year/patient) • Plasma half-life of ~9 hrs • Undergoes glucuronidation + renal clearance • Does not inhibit nor induce CYP enzymes Clinical: • HIV integrase inhibitor for use in combination with other antiretroviral agents for HAART resistant HIV-1 infection • Also recommended for initial HAART • Resistance observed by mutation of catalytic site Pharmacovigilance: • Diarrhea, nausea, headache and pyrexia

Dolutegravir and Elvitegravir and Bictegravir and Cabotegravir All are HIV integrase inhibitors Used in combination with protease inhibitors. Dolutegravir (approved 2013) Found to be 7% more effective than raltegravir in clinical trials. Effective in patients resistant to raltegravir Elvitegravir (approved in 2015) Metabolized by CYP 3 A 4 Bictegravir (approved in 2018) Metabolized by CYP 3 A 4 Cabotegravir (approved in 2021) Metabolized by CYP 3 A 4
![Cobicistat Tybost Inhibits liver enzymes that metabolize other antiviral drugs Potent inhibitor of Cobicistat [Tybost] Inhibits liver enzymes that metabolize other antiviral drugs – Potent inhibitor of](https://slidetodoc.com/presentation_image_h2/a980f64860d6c103ee7221bbb79308bf/image-55.jpg)
Cobicistat [Tybost] Inhibits liver enzymes that metabolize other antiviral drugs – Potent inhibitor of CYP 3 A 4 – Also inhibits intestinal transport proteins to increase absorption of other drugs Allows lower doses of these other drugs to achieve higher concentrations for longer periods, increasing antiviral activity while reducing side effects Combos: – elvitegravir/cobicistat/emtricitabine/tenofo vir disoproxil – elvitegravir/cobicistat/emtricitabine/tenofo vir alafenamide – darunavir/cobicistat – atazanavir/cobicistat

INHBITORS OF OF INFLUENZA NEURAMINIDASE

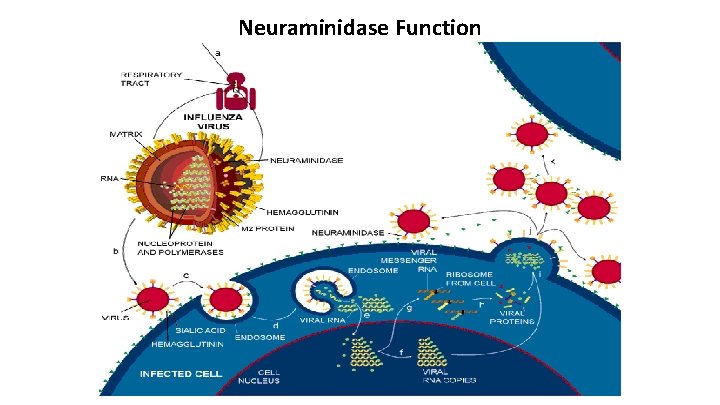
Neuraminidase Function

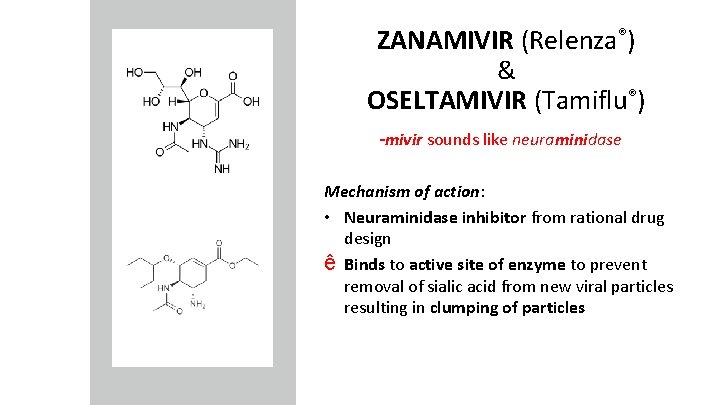
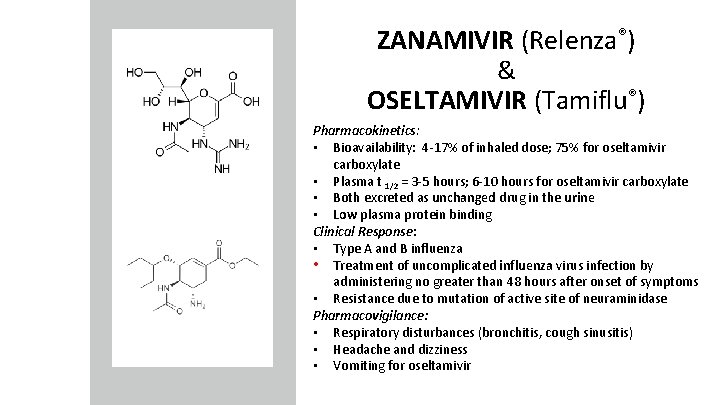
ZANAMIVIR (Relenza®) & OSELTAMIVIR (Tamiflu®) -mivir sounds like neuraminidase Mechanism of action: • Neuraminidase inhibitor from rational drug design ê Binds to active site of enzyme to prevent removal of sialic acid from new viral particles resulting in clumping of particles

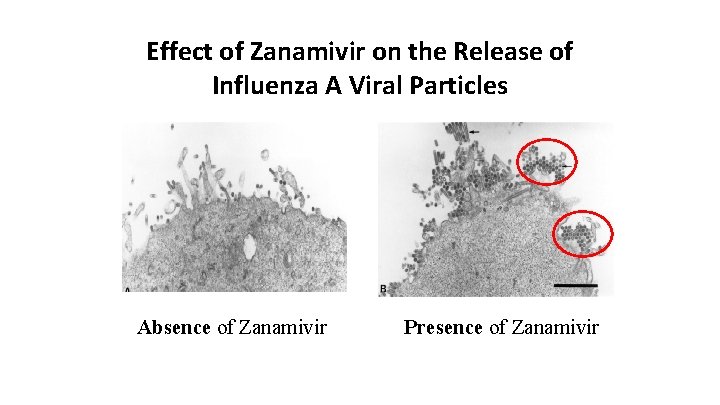
Effect of Zanamivir on the Release of Influenza A Viral Particles Absence of Zanamivir Presence of Zanamivir

ZANAMIVIR (Relenza®) & OSELTAMIVIR (Tamiflu®) Pharmacokinetics: • Bioavailability: 4 -17% of inhaled dose; 75% for oseltamivir carboxylate • Plasma t 1/2 = 3 -5 hours; 6 -10 hours for oseltamivir carboxylate • Both excreted as unchanged drug in the urine • Low plasma protein binding Clinical Response: • Type A and B influenza • Treatment of uncomplicated influenza virus infection by administering no greater than 48 hours after onset of symptoms • Resistance due to mutation of active site of neuraminidase Pharmacovigilance: • Respiratory disturbances (bronchitis, cough sinusitis) • Headache and dizziness • Vomiting for oseltamivir

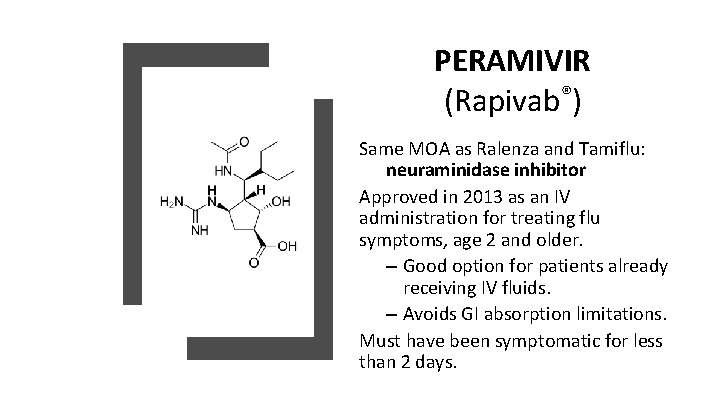
PERAMIVIR (Rapivab®) Same MOA as Ralenza and Tamiflu: neuraminidase inhibitor Approved in 2013 as an IV administration for treating flu symptoms, age 2 and older. – Good option for patients already receiving IV fluids. – Avoids GI absorption limitations. Must have been symptomatic for less than 2 days.

NON-SPECIFIC VIRAL INHIBITORS: HUMAN INTERFERONS

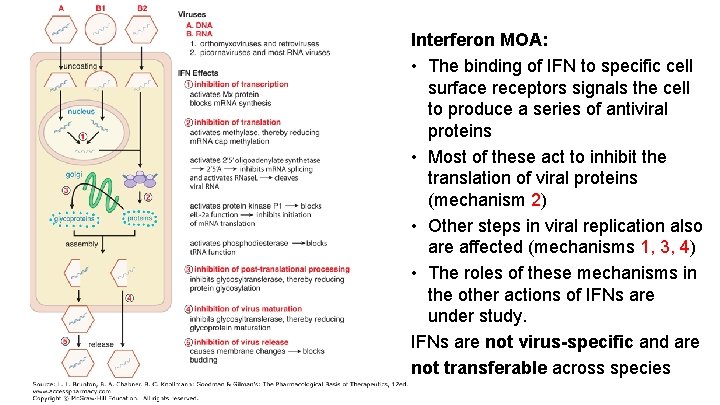
Interferon MOA: • The binding of IFN to specific cell surface receptors signals the cell to produce a series of antiviral proteins • Most of these act to inhibit the translation of viral proteins (mechanism 2) • Other steps in viral replication also are affected (mechanisms 1, 3, 4) • The roles of these mechanisms in the other actions of IFNs are under study. IFNs are not virus-specific and are not transferable across species

Clinical Response: HUMAN INTERFERONS • Main clinical use: chronic active hepatitis B & C (combined with ribavirin) • Useful for varicella-zoster virus (chicken pox; shingles) infections in immuno-compromised patients • Papillomavirus (warts) infections are also susceptible • Various types suggest many applications

Pharmacokinetics: HUMAN INTERFERONS • Powders for reconstitution as solutions for s. c. or i. m. administration • Undergo glomerular filtration and proteolytic degradation in renal tubules • Half life ranges from 2 -8 hrs. depending upon route and formulation Pharmacovigilance: • Typically a “flu-like” syndrome • Transient elevation of hepatic enzymes • Alpha-interferons are abortifacient in primates and should not be used in pregnancy

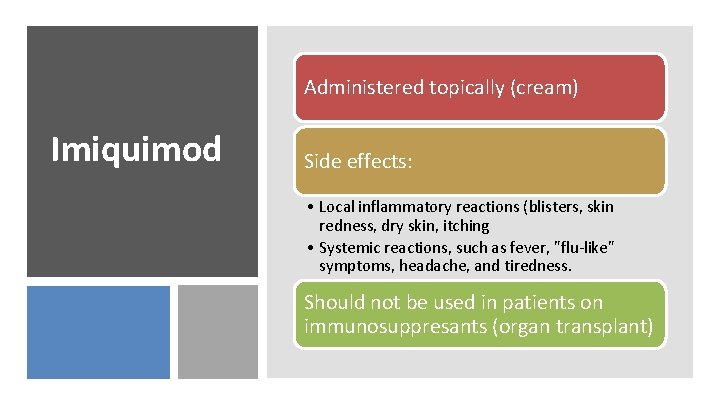
Immunomodulatory agent that is effective for topical treatment of dermatologic conditions associated with DNA virus infections. Imiquimod • Approved for treatment of genital warts and basal cell carcinoma • Works on regular warts, also Induces local IFN-α, -β, and -γ and TNFα responses and causes reductions in viral load. May act via Toll-like receptor 7 (TLR 7) signaling.

Administered topically (cream) Imiquimod Side effects: • Local inflammatory reactions (blisters, skin redness, dry skin, itching • Systemic reactions, such as fever, "flu-like" symptoms, headache, and tiredness. Should not be used in patients on immunosuppresants (organ transplant)

Hepatitis C Drugs

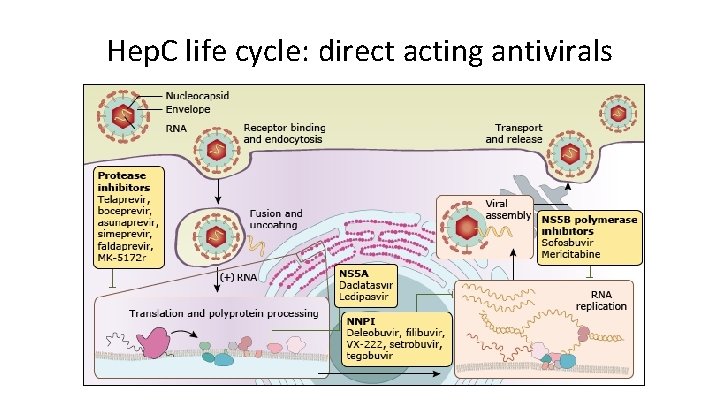
Hep. C life cycle: direct acting antivirals

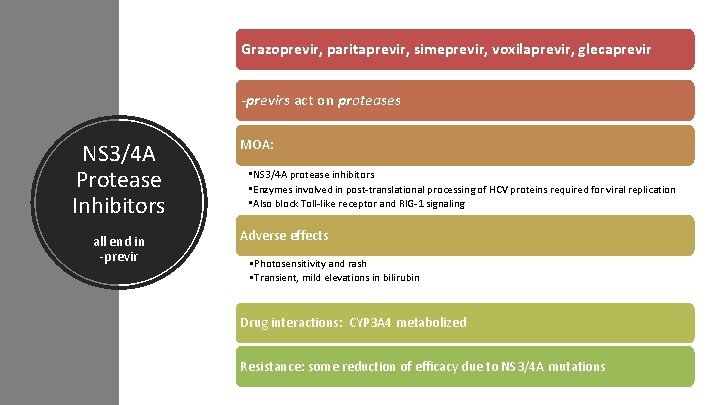
Grazoprevir, paritaprevir, simeprevir, voxilaprevir, glecaprevir -previrs act on proteases NS 3/4 A Protease Inhibitors all end in -previr MOA: • NS 3/4 A protease inhibitors • Enzymes involved in post-translational processing of HCV proteins required for viral replication • Also block Toll-like receptor and RIG-1 signaling Adverse effects • Photosensitivity and rash • Transient, mild elevations in bilirubin Drug interactions: CYP 3 A 4 metabolized Resistance: some reduction of efficacy due to NS 3/4 A mutations

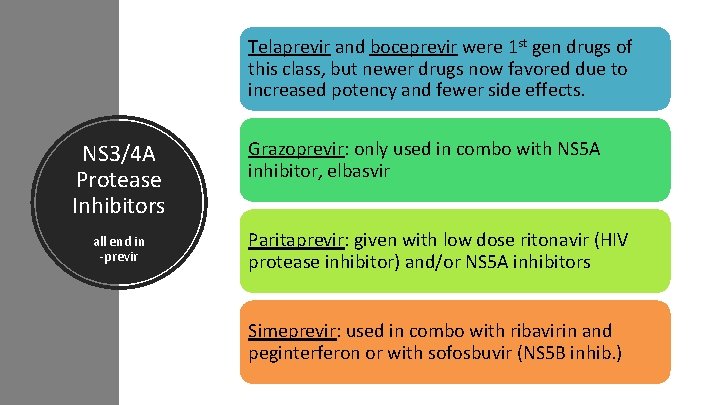
Telaprevir and boceprevir were 1 st gen drugs of this class, but newer drugs now favored due to increased potency and fewer side effects. NS 3/4 A Protease Inhibitors all end in -previr Grazoprevir: only used in combo with NS 5 A inhibitor, elbasvir Paritaprevir: given with low dose ritonavir (HIV protease inhibitor) and/or NS 5 A inhibitors Simeprevir: used in combo with ribavirin and peginterferon or with sofosbuvir (NS 5 B inhib. )

Daclatasvir, ledipasvir, ombitasvir, velpatasvir, elbasvir, pibrentasvir NS 5 A inhibitors all end in -asvir is NS 5 A backwards MOA: NS 5 A inhibitors • NS 5 A plays an unknown role in assembly of HCV virus All used in combinations • Daclatasvir-sofosbuvir, Ledipasvir-sofosbuvir, Ombitasvir-paritaprevir, Ombitasvir– ritonavir, Elbasvir-grazoprevir, Velpatasvir-sofosbuvir-voxilaprevir. Adverse effects: Minor (headache, fatigue, nausea) Drug interactions: CYP 3 A 4 metabolized, p-glycoprotein inhibitor (reduce digoxin dose) Resistance: NS 5 A polymorphisms can confer resistance

Sofosbuvir, dasabuvir NS 5 B Inhibitors all end in -buvir translates to NS 5 B MOA: NS 5 B RNA polymerase inhibitors • Sofos: nucleoside inhibitor of the polymerase • Potent • Not genotype specific • Dasa: non-nucleoside inhibitor (allosteric) • Less potent • Genotype specific • Both lead to inhibition of translation and blockade of viral replication Used only in combination with other antiviral drugs Adverse effects: Minor (headache, fatigue, nausea) Drug interactions: p-glycoprotein substrate (reduce dose when given with P-gp inducers).

Hep. B Drugs

Adefovir • MOA: acyclic nucleotide, reverse transcriptase inhibitor – Nucleotide analog – Interferes with HBV RNAdependent DNA polymerase • Adverse effects: lactic acidosis and severe hepatomegaly • Drug interactions: do not combine with Tenovir.

Entecavir • MOA: inhibits HBV polymerase to block reverse transcriptase activity – guanosine analog that gets phosphorylated intracellularly and competes with natural substrates • Adverse effects: lactic acidosis and severe hepatomegaly – Peripheral edema, ascites, increased serum ALT and creatinine, fever • Drug interactions: Orlistat (obesity drug) may increase serum concentration of entecavir

Study your drugs! MAKE FLASHCARDS ON QUIZLET DRUG SUMMARY STUDY SHEET POSTED TO CANVAS.
 Antiviral ajan
Antiviral ajan Second antiviral pill
Second antiviral pill Czajkowski opery
Czajkowski opery Elinor ostrom social ecological systems
Elinor ostrom social ecological systems Exel
Exel Edu.sharif.edu
Edu.sharif.edu Antipseudomonal drugs
Antipseudomonal drugs Concepts of primary health care
Concepts of primary health care Digoxin contraindications
Digoxin contraindications Hemostatis adalah
Hemostatis adalah Acidic drug example
Acidic drug example Mechanism of action of parasympathomimetic drugs
Mechanism of action of parasympathomimetic drugs Seizure threshold meaning
Seizure threshold meaning Vegetable drugs
Vegetable drugs Antispasmodic classification
Antispasmodic classification Differentiate between organised and unorganised crude drugs
Differentiate between organised and unorganised crude drugs Intracameral pilocarpine
Intracameral pilocarpine Csa schedule
Csa schedule Balanced anesthesia components
Balanced anesthesia components Tocolytic drugs
Tocolytic drugs Wendy breastfeeding drugs
Wendy breastfeeding drugs Drugs used in pregnancy
Drugs used in pregnancy Classify anticholinergic drugs
Classify anticholinergic drugs Drugs addict enkawl dan
Drugs addict enkawl dan Storage and maintenance of drugs
Storage and maintenance of drugs Classification of anticholinergic drugs
Classification of anticholinergic drugs Mechanism of antifungal drugs
Mechanism of antifungal drugs Granulopoiesis
Granulopoiesis Pharmacology of drugs acting on respiratory system
Pharmacology of drugs acting on respiratory system Drugs that don't cross placenta mnemonic
Drugs that don't cross placenta mnemonic Thioxanthenes drugs examples
Thioxanthenes drugs examples Neurotransmitters and drugs
Neurotransmitters and drugs Galanthamini hydrobromidum
Galanthamini hydrobromidum Anti diarrheal drugs list
Anti diarrheal drugs list Emergency drugs in crash cart
Emergency drugs in crash cart Antigout drugs classification
Antigout drugs classification Driver training toolbox
Driver training toolbox Antiarrhythmic drugs classification
Antiarrhythmic drugs classification Antidiabetic drugs classification
Antidiabetic drugs classification Belladonna alkaloids drugs
Belladonna alkaloids drugs Cardiotropic drugs
Cardiotropic drugs Section 17-3 practice commonly abused drugs
Section 17-3 practice commonly abused drugs Chapter 19 medicines and drugs vocabulary practice
Chapter 19 medicines and drugs vocabulary practice Anticancer drugs classification
Anticancer drugs classification Blocking muscarinic receptors would
Blocking muscarinic receptors would Liothyronine
Liothyronine Drugs definition
Drugs definition Bugs need drugs
Bugs need drugs Sympathomimetic agents
Sympathomimetic agents Lithium mechanism of action
Lithium mechanism of action Chapter 24 heart failure drugs
Chapter 24 heart failure drugs Irbesartan
Irbesartan Sympathetic drugs
Sympathetic drugs Resisting pressure to abuse drugs is a responsible
Resisting pressure to abuse drugs is a responsible Sulfonamides classification
Sulfonamides classification Module 25 psychoactive drugs
Module 25 psychoactive drugs Site:slidetodoc.com
Site:slidetodoc.com Antianginal drugs
Antianginal drugs Inotropic drugs
Inotropic drugs Scientific working group for the analysis of seized drugs
Scientific working group for the analysis of seized drugs Antidiuretic drugs
Antidiuretic drugs Metabolic changes of drugs and related organic compounds
Metabolic changes of drugs and related organic compounds Alpha blockers
Alpha blockers Look alike sound alike drugs
Look alike sound alike drugs Bell ringer weed
Bell ringer weed Antithyroid drugs
Antithyroid drugs Bronchodilators
Bronchodilators Gugulsterone
Gugulsterone Etanecept
Etanecept Anticholinergic drugs classification
Anticholinergic drugs classification Tractocil
Tractocil Bronchodilator drugs
Bronchodilator drugs Anticholinergic drugs mechanism of action
Anticholinergic drugs mechanism of action Hepatotoxic drugs
Hepatotoxic drugs Parasympathomimetic drugs
Parasympathomimetic drugs Possibly armed probably not
Possibly armed probably not Denk pharma products
Denk pharma products Antidiarrheal drugs classification
Antidiarrheal drugs classification