Scientific Working Group for the Analysis of Seized




















- Slides: 23

Scientific Working Group for the Analysis of Seized Drugs Update and NAS Report Discussion by Scott R. Oulton, SWGDRUG Secretariat

SWGDRUG UPDATE The core committee voted to adopt the uncertainty document in July 2008 q What Next? – Four Active Subcommittees § 1) Uncertainty Subcommittee o Develop Supplemental Documents § 2) Education and Training Subcommittee o Devise Comprehensive Training Program § 3) Editorial/Communications Subcommittee o Revise/Edit Current SWGDRUG Recommendations § 4) Coordination with CLIC o Developing standards for the ID of inorganics q

UNCERTAINTY SUBCOMMITTEE q Working on Supplementary Documents to include “real world” examples § Supplementary Documents Intended to be a resource for those implementing recommendations o Not all inclusive, many ways to implement recommendations o q Goals § To cover as many laboratory situations as possible and make them clear and concise § Qualitative and quantitative methods addressed § Provide references

UNCERTAINTY SUBCOMMITTEE q Two general approaches § “Bottom-up” uncertainty budget § “Top-down” incorporating method validation and continuing QA/QC such as control charts q Examples adapted from working laboratory protocols q Examples posted in July 2009 and are being vetted through professional metrologists q Goal is to have documents adopted in January 2010

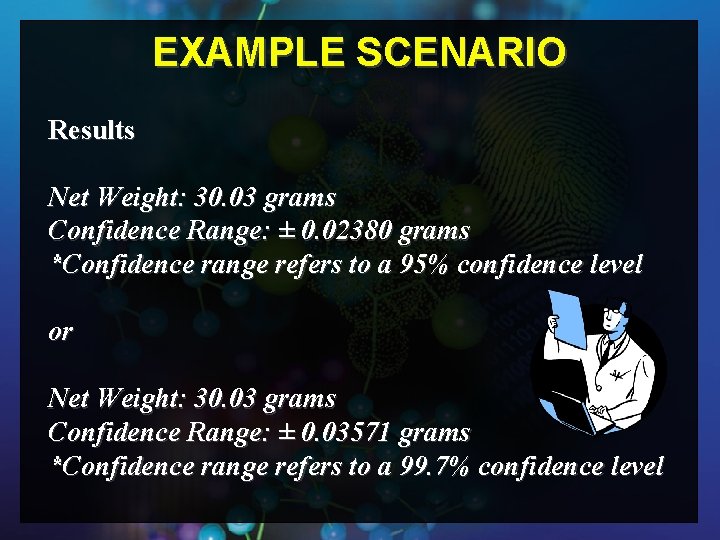
EXAMPLE SCENARIO Determine net weight of a white powder received in a plastic bag using a top loading balance. The following conditions apply: § The operator is competent on the use of the balance § The balance is calibrated and certified § The balance is performing within manufacturer specifications § The balance operates at an ambient temperature varying ± 5 °C q The weight recorded for the powder, determined by placing the material inside a tared weighing dish, is 30. 03 grams q

FACTORS TO CONSIDER q Readability q Repeatability q Linearity q Sensitivity q Sample loss in transfer: The uncertainty associated with sample loss is, for practical purposes, indeterminate and irrelevant

EXAMPLE SCENARIO Results Net Weight: 30. 03 grams Confidence Range: ± 0. 02380 grams *Confidence range refers to a 95% confidence level or Net Weight: 30. 03 grams Confidence Range: ± 0. 03571 grams *Confidence range refers to a 99. 7% confidence level

WHAT NEXT? q Second example covers additive weights (multiple exhibits); similar level of detail q Quantitative methods are in early form but in essence capture uncertainty by means of method validation, controls and other QC protocols q Address single lab and multi-lab organizations

EXISTING TRAINING PROGRAM q 4. 2 Topic areas in the training program will include, as a minimum, the following: § Relevant background information on drugs of abuse (e. g. , status of control and chemical and physical characteristics) § Techniques, methodologies and instrumentation utilized in the examination of seized drug samples and related materials § Quality assurance § Expert /Court testimony and legal requirements § Laboratory policy and procedures (e. g. , sampling, uncertainty, evidence handling, safety and security) as they relate to the examination of seized drug samples and related materials.

EDUCATION AND TRAINING SUBCOMMITTEE q Task – Devise comprehensive training program q Coordinate efforts with ENFSI Drugs Working Group q Develop on-line program § Downloadable § Hypertext Linking § References

EDITORIAL COMMITTEE q Goal – Revise existing document to: § Harmonize terminology § Correct grammar § Add references § Link sections § Correct sections in conflict § Clarify recommendations as appropriate

EDITORIAL COMMITTEE q 2 Education and experience for analysts § Removed: o a bachelor’s degree (or equivalent, generally a three to four year post-secondary or tertiary degree) in a natural science or in other sciences relevant to the analysis of seized drugs… OR o by January 1, 2005, a minimum of five (5) years practical experience in the area of seized drug analysis… § Revised: o All new analysts shall have at least a bachelor’s degree (or equivalent, generally a three to four year post-secondary degree) in a natural/physical science.

EDITORIAL COMMITTEE q 3 Continuing professional development § As Written: o Contact is defined as face-to-face interaction with an instructor or trainer in a classroom or laboratory setting. It does not include self-paced learning or distance education where the instructor has no active interaction with the student. § As Revised: o 3. 4 Training can be either face-to-face interaction with an instructor, distance learning, self-directed or computer based. Ø Added: current literature review

EDITORIAL COMMITTEE 11 Analytical method validation and verification § As Written: o 11. 1 Method validation is required to demonstrate that methods are suitable for their intended purpose. Ø 11. 1. 1 For qualitative analysis, the parameters that need to be checked are selectivity, limit of detection and reproducibility. Ø 11. 1. 2 Minimum acceptability criteria should be described along with means for demonstrating compliance. Ø 11. 1. 3 Validation documentation is required. o 11. 2 Laboratories adopting methods validated elsewhere should verify these methods and establish their own limits of detection and reproducibility. § As Revised: o Method validation is required to demonstrate that methods are suitable for their intended purpose (see PART IV B – Validation).

EDITORIAL COMMITTEE q Added hyperlinks and references to UNCERTAINTY throughout document q Added hyperlinks and references to VALIDATION throughout document q Added “Shall” in place of “Should” in several locations (conduct, ethics, education, etc. ) q Category A now includes: X-Ray Diffractometry

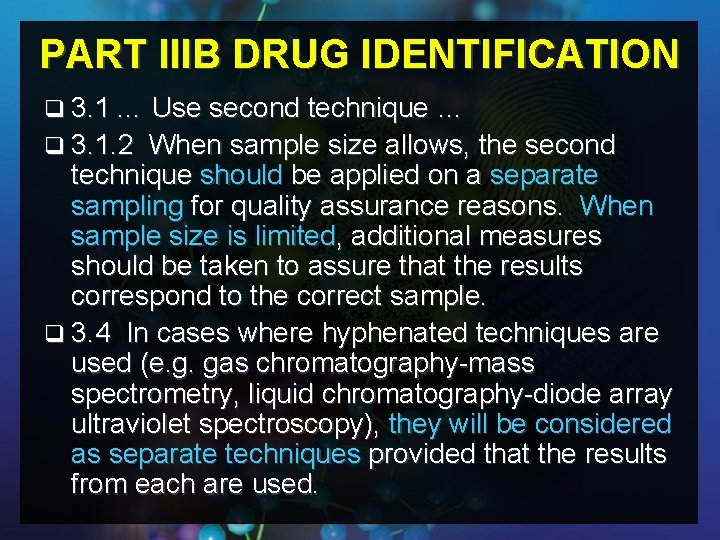
PART IIIB DRUG IDENTIFICATION q 3. 1 … Use second technique … q 3. 1. 2 When sample size allows, the second technique should be applied on a separate sampling for quality assurance reasons. When sample size is limited, additional measures should be taken to assure that the results correspond to the correct sample. q 3. 4 In cases where hyphenated techniques are used (e. g. gas chromatography-mass spectrometry, liquid chromatography-diode array ultraviolet spectroscopy), they will be considered as separate techniques provided that the results from each are used.

PART IIIB DRUG IDENTIFICATION q Problem § If two samplings important, why have different procedure for trace samples? § Misinterpretation of 3. 4, hyphenated techniques do not offer second sampling q Solution § Revise section to emphasize quality assurance step Second sampling o Procedural blank o Witnessing o

PART IIIB DRUG IDENTIFICATION q Solution – As Written § The laboratory shall employ quality assurance measures to ensure the results correspond to the exhibit. Example measures are: o o o the use of two separate samplings sample identification procedures such as barcoding and witness checks good laboratory practices (e. g. , positive and negative controls, one sample opened at a time, procedural blanks)

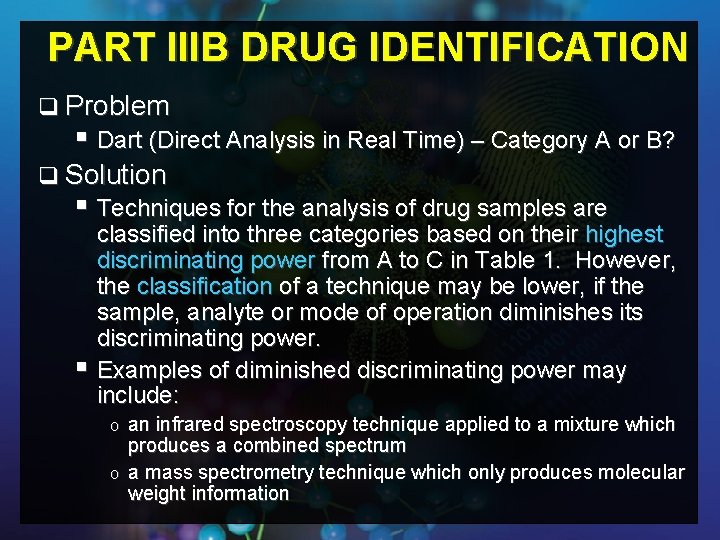
PART IIIB DRUG IDENTIFICATION q Problem § Dart (Direct Analysis in Real Time) – Category A or B? q Solution § Techniques for the analysis of drug samples are classified into three categories based on their highest discriminating power from A to C in Table 1. However, the classification of a technique may be lower, if the sample, analyte or mode of operation diminishes its discriminating power. § Examples of diminished discriminating power may include: an infrared spectroscopy technique applied to a mixture which produces a combined spectrum o a mass spectrometry technique which only produces molecular weight information o

CORE COMMITTEE • DEA – Nelson Santos (Chair) • • Secretariat – Scott Oulton (non-voting) FBI - Eileen Waninger ASCLD – Garth Glassburg NIST - Susan Ballou ASTM and NEAFS- Jack Mario Educator – Dr. Chris Tindall Educator – Dr. Suzanne Bell

CORE COMMITTEE • CAC & NWAFS - Jerry Massetti • • MAFS - Richard Paulas MAAFS - Linda Jackson SAFS – Christian Matchett Toxicology – Dr. Robert Powers

CORE COMMITTEE • Canada - Richard Laing • • • Japan – Mr. Osamu Ohtsuru United Kingdom - Dr. Sylvia Burns Australia - Catherine Quinn Germany - Dr. Udo Zerell ENFSI - Dr. Michael Bovens UNODC - Dr. Iphigenia Naidis

Visit us at: www. swgdrug. org
 Scientific working group for the analysis of seized drugs
Scientific working group for the analysis of seized drugs Panic seized the writer passive voice
Panic seized the writer passive voice No temptation has seized you
No temptation has seized you No temptation
No temptation Panic seized the writer passive voice
Panic seized the writer passive voice Hard work vs smart work
Hard work vs smart work Principle of hot working process
Principle of hot working process Hot working and cold working difference
Hot working and cold working difference Machining operations
Machining operations Contoh hot working
Contoh hot working Information gathered during an experiment
Information gathered during an experiment How is a scientific law different from a scientific theory?
How is a scientific law different from a scientific theory? Crisis communication working group
Crisis communication working group Oecd working group on bribery
Oecd working group on bribery Cells working together form
Cells working together form Understanding work teams
Understanding work teams Coloured gemstones working group
Coloured gemstones working group A group vs a team
A group vs a team Awgesc asean
Awgesc asean Asean working group on climate change
Asean working group on climate change Swiss nwg
Swiss nwg Power curve working group
Power curve working group Group of cells working together
Group of cells working together Plug working group
Plug working group