Anticoagulation post TAVR How Should We Find the




- Slides: 22

Anticoagulation post – TAVR: How Should We Find the Optimal Protocol ? Alec Vahanian Bichat Hospital University Paris VII

Alec Vahanian MD <Type of Relationship>: <Company 1> Consultancy Edwards Life Sciences

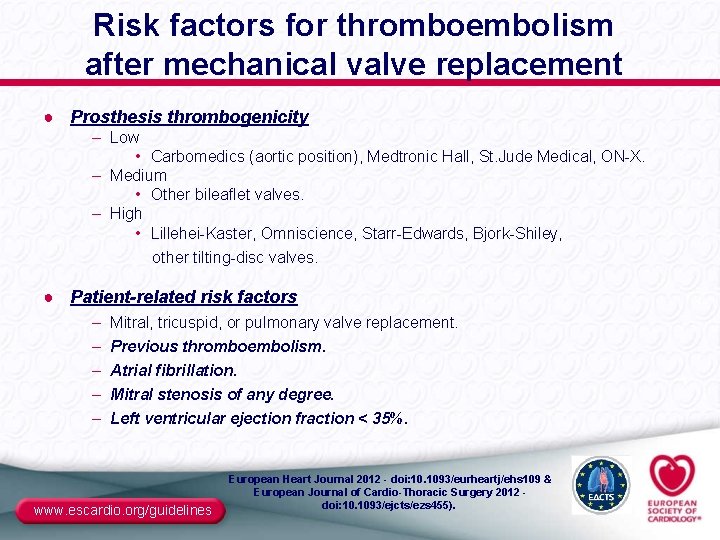
Risk factors for thromboembolism after mechanical valve replacement ● Prosthesis thrombogenicity – Low • Carbomedics (aortic position), Medtronic Hall, St. Jude Medical, ON-X. – Medium • Other bileaflet valves. – High • Lillehei-Kaster, Omniscience, Starr-Edwards, Bjork-Shiley, other tilting-disc valves. ● Patient-related risk factors – – – Mitral, tricuspid, or pulmonary valve replacement. Previous thromboembolism. Atrial fibrillation. Mitral stenosis of any degree. Left ventricular ejection fraction < 35%. www. escardio. org/guidelines European Heart Journal 2012 - doi: 10. 1093/eurheartj/ehs 109 & European Journal of Cardio-Thoracic Surgery 2012 doi: 10. 1093/ejcts/ezs 455).

Post-Operative Antithrombotic Therapy after Bioprosthesis Implantation • 28 series • Limitations – Few comparatives studies – Mix of aortic and mitral bioprosthesis – Confounding factors – Only 2 prospectives series • One non-randomised comparative series • Only one randomised trial (Nowell et al. Eur J Cariothoracic Surg 2007; 31: 578 -85)

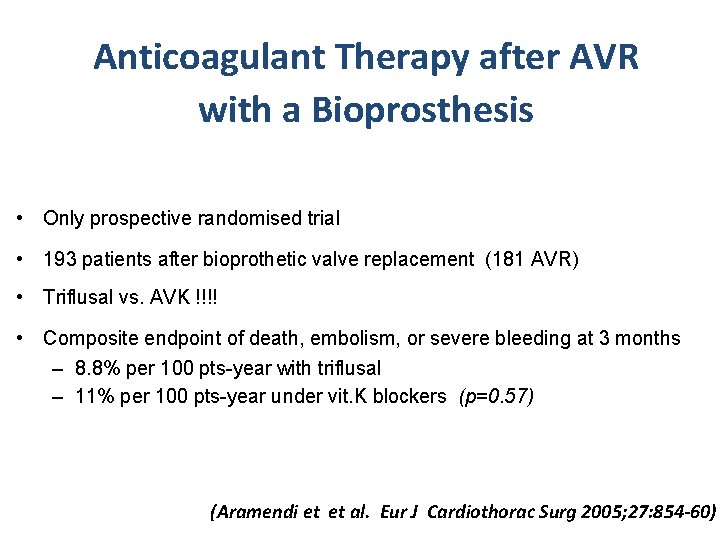
Anticoagulant Therapy after AVR with a Bioprosthesis • Only prospective randomised trial • 193 patients after bioprothetic valve replacement (181 AVR) • Triflusal vs. AVK !!!! • Composite endpoint of death, embolism, or severe bleeding at 3 months – 8. 8% per 100 pts-year with triflusal – 11% per 100 pts-year under vit. K blockers (p=0. 57) (Aramendi et et al. Eur J Cardiothorac Surg 2005; 27: 854 -60)

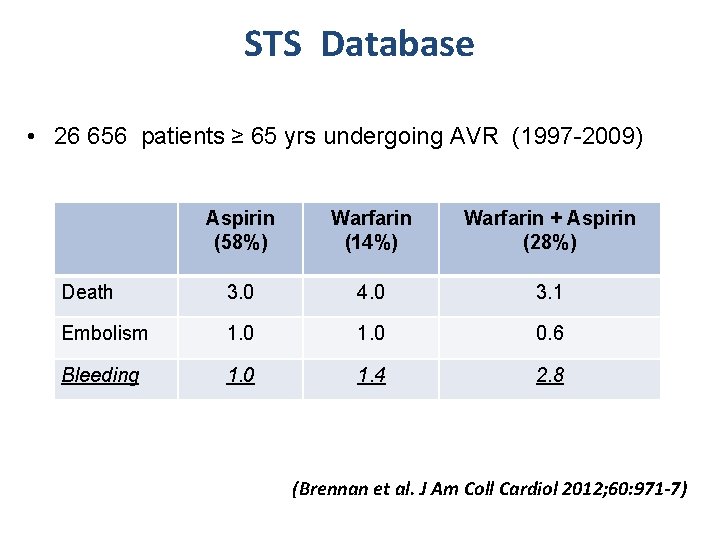
STS Database • 26 656 patients ≥ 65 yrs undergoing AVR (1997 -2009) Aspirin (58%) Warfarin (14%) Warfarin + Aspirin (28%) Death 3. 0 4. 0 3. 1 Embolism 1. 0 0. 6 Bleeding 1. 0 1. 4 2. 8 (Brennan et al. J Am Coll Cardiol 2012; 60: 971 -7)

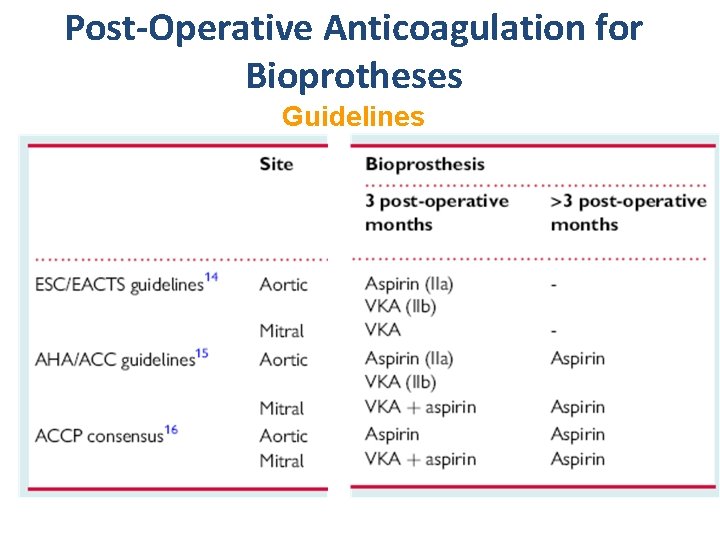
Post-Operative Anticoagulation for Bioprotheses Guidelines

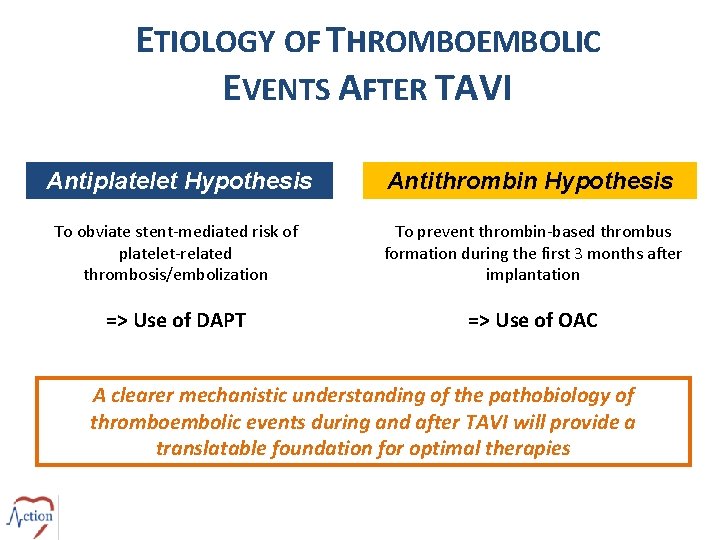
ETIOLOGY OF THROMBOEMBOLIC EVENTS AFTER TAVI Antiplatelet Hypothesis Antithrombin Hypothesis To obviate stent-mediated risk of platelet-related thrombosis/embolization To prevent thrombin-based thrombus formation during the first 3 months after implantation => Use of DAPT => Use of OAC A clearer mechanistic understanding of the pathobiology of thromboembolic events during and after TAVI will provide a translatable foundation for optimal therapies

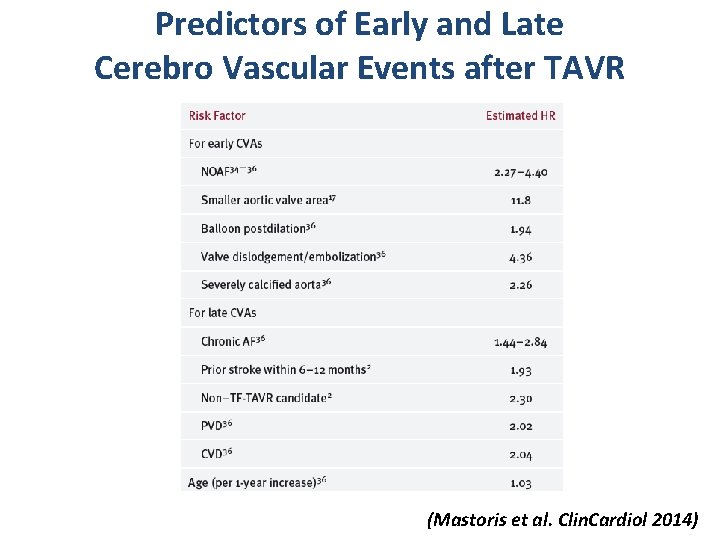
Predictors of Early and Late Cerebro Vascular Events after TAVR (Mastoris et al. Clin. Cardiol 2014)

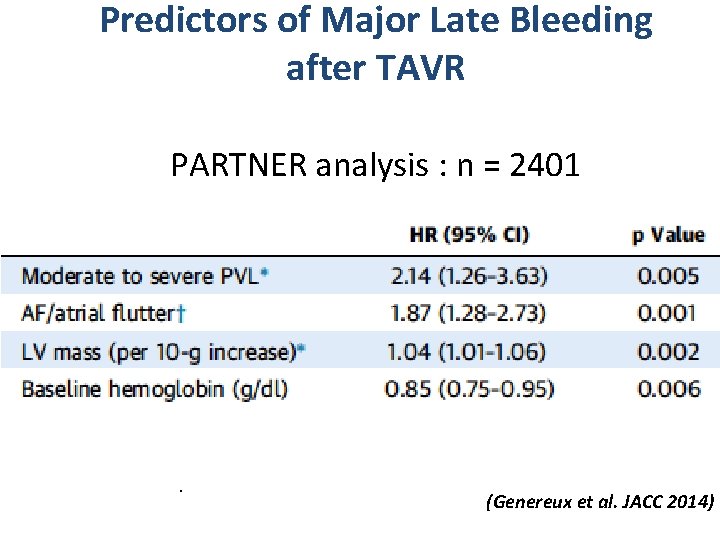
Predictors of Major Late Bleeding after TAVR PARTNER analysis : n = 2401 . (Genereux et al. JACC 2014)

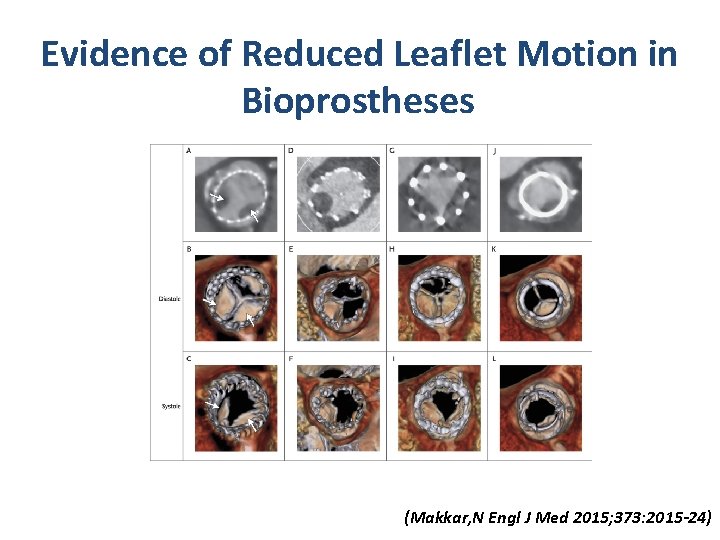
Evidence of Reduced Leaflet Motion in Bioprostheses. Makkar RR et al. N Engl J Med 2015; 373: 2015 -2024 (Makkar, N Engl J Med 2015; 373: 2015 -24)

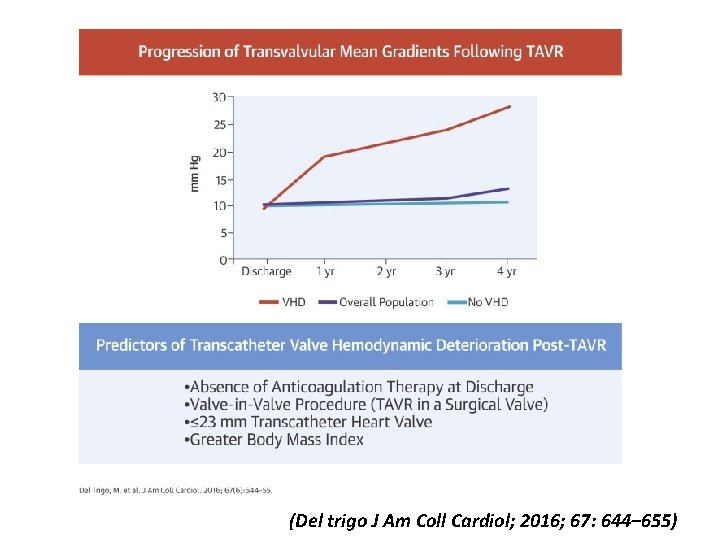
(Del trigo J Am Coll Cardiol; 2016; 67: 644– 655)

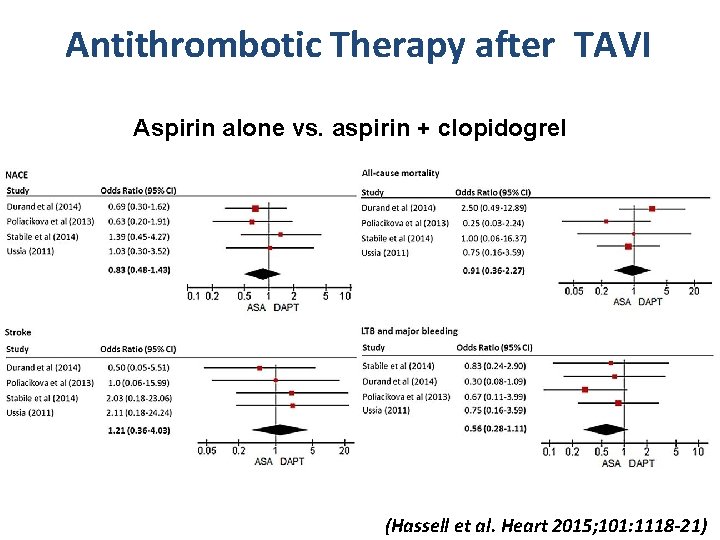
Antithrombotic Therapy after TAVI Aspirin alone vs. aspirin + clopidogrel (Hassell et al. Heart 2015; 101: 1118 -21)

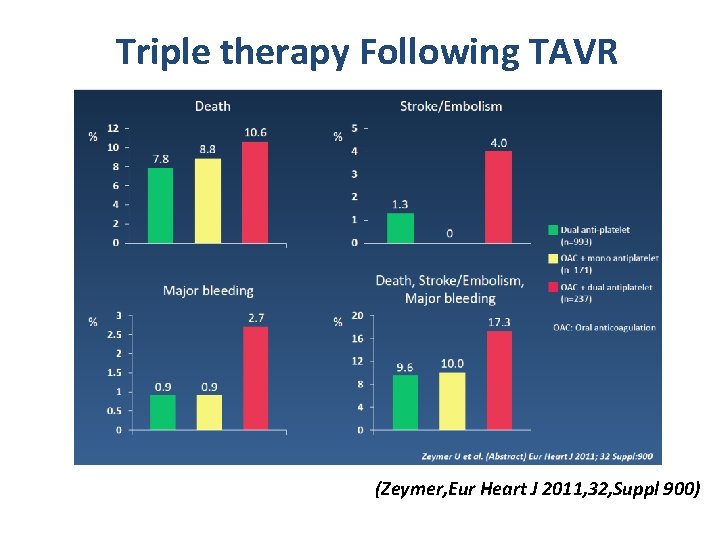
Triple therapy Following TAVR (Zeymer, Eur Heart J 2011, 32, Suppl 900)

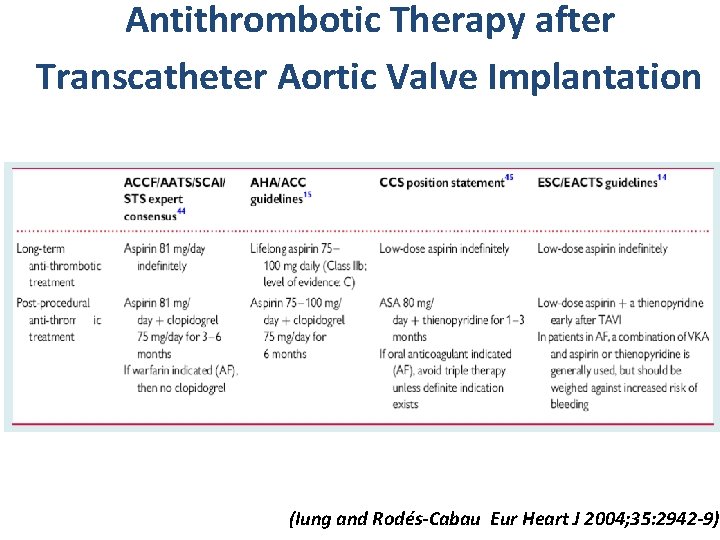
Antithrombotic Therapy after Transcatheter Aortic Valve Implantation (Iung and Rodés-Cabau Eur Heart J 2004; 35: 2942 -9))

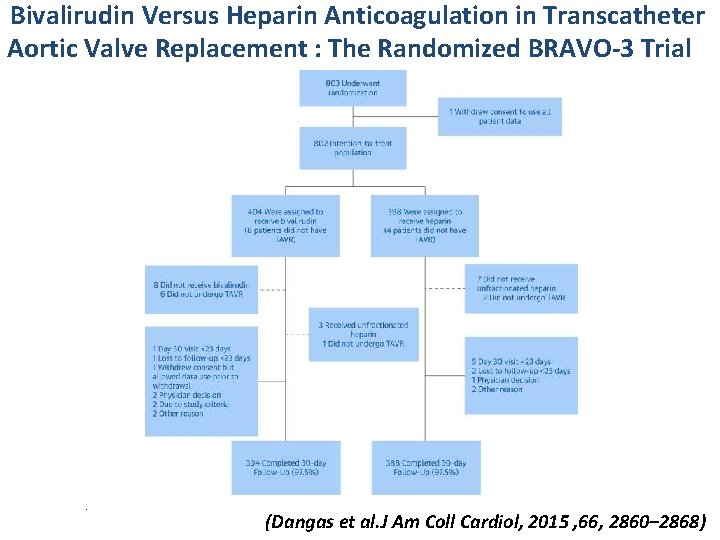
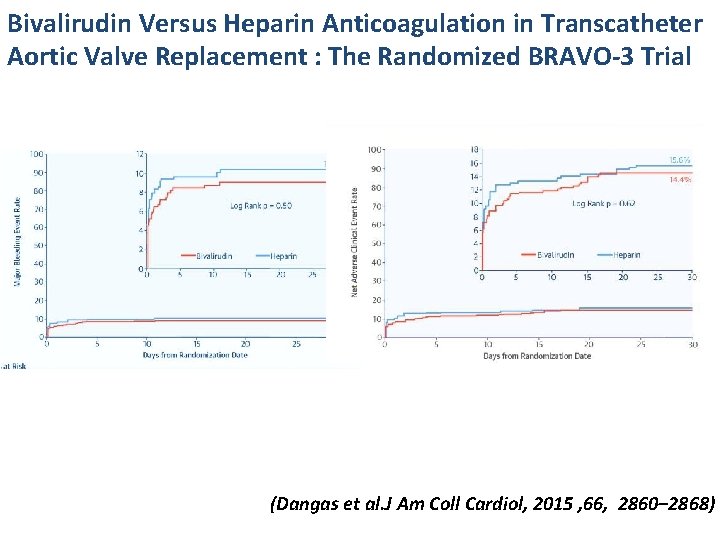
Bivalirudin Versus Heparin Anticoagulation in Transcatheter Aortic Valve Replacement : The Randomized BRAVO-3 Trial . (Dangas et al. J Am Coll Cardiol, 2015 , 66, 2860– 2868)

Bivalirudin Versus Heparin Anticoagulation in Transcatheter Aortic Valve Replacement : The Randomized BRAVO-3 Trial (Dangas et al. J Am Coll Cardiol, 2015 , 66, 2860– 2868)

Direct oral anticoagulants • They are labelled only for non-valvular atrial fibrillation • “No satisfactory or uniform definition of these terms exists. ” (2012 ESC Guidelines on Atrial Fibrillation) • Available evidence: ─ AS, AR, MR: data on rivaroxaban and apixaban ─ MS, bioprostheses: no data ─ Mechanical prostheses: contra-indication 19

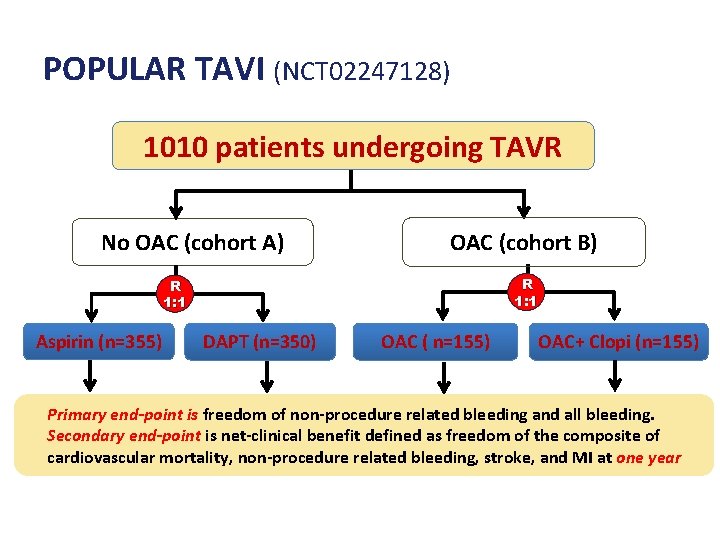
POPULAR TAVI (NCT 02247128) 1010 patients undergoing TAVR No OAC (cohort A) OAC (cohort B) R 1: 1 Aspirin (n=355) DAPT (n=350) OAC ( n=155) OAC+ Clopi (n=155) Primary end-point is freedom of non-procedure related bleeding and all bleeding. Secondary end-point is net-clinical benefit defined as freedom of the composite of cardiovascular mortality, non-procedure related bleeding, stroke, and MI at one year

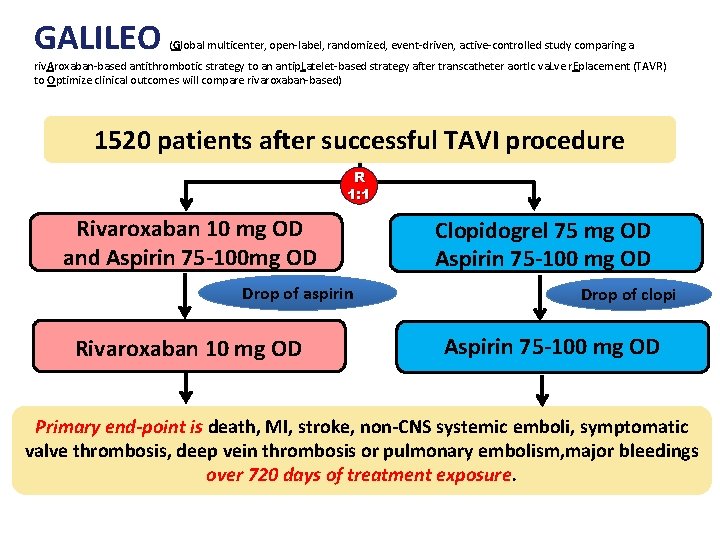
GALILEO (Global multicenter, open-label, randomized, event-driven, active-controlled study comparing a riv. Aroxaban-based antithrombotic strategy to an antip. Latelet-based strategy after transcatheter aort. Ic va. Lve r. Eplacement (TAVR) to Optimize clinical outcomes will compare rivaroxaban-based) 1520 patients after successful TAVI procedure R 1: 1 Rivaroxaban 10 mg OD and Aspirin 75 -100 mg OD Drop of aspirin Rivaroxaban 10 mg OD Clopidogrel 75 mg OD Aspirin 75 -100 mg OD Drop of clopi Aspirin 75 -100 mg OD Primary end-point is death, MI, stroke, non-CNS systemic emboli, symptomatic valve thrombosis, deep vein thrombosis or pulmonary embolism, major bleedings over 720 days of treatment exposure.

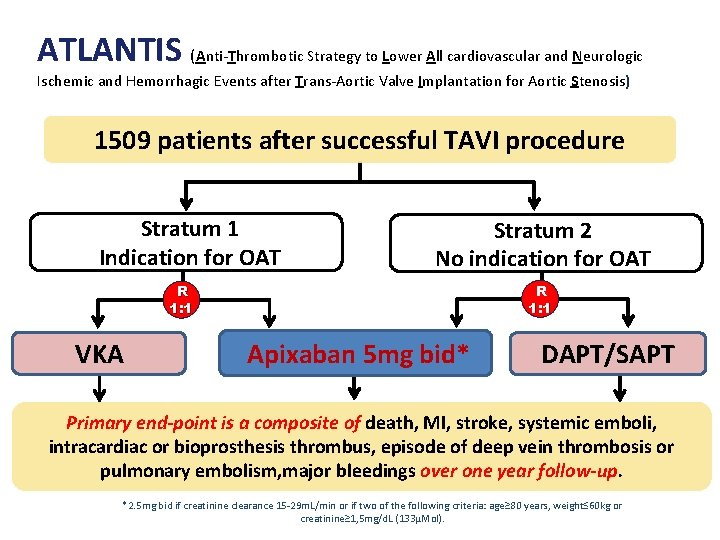
ATLANTIS (Anti-Thrombotic Strategy to Lower All cardiovascular and Neurologic Ischemic and Hemorrhagic Events after Trans-Aortic Valve Implantation for Aortic Stenosis) 1509 patients after successful TAVI procedure Stratum 1 Indication for OAT Stratum 2 No indication for OAT R 1: 1 VKA Apixaban 5 mg bid* DAPT/SAPT Primary end-point is a composite of death, MI, stroke, systemic emboli, intracardiac or bioprosthesis thrombus, episode of deep vein thrombosis or pulmonary embolism, major bleedings over one year follow-up. *2. 5 mg bid if creatinine clearance 15 -29 m. L/min or if two of the following criteria: age≥ 80 years, weight≤ 60 kg or creatinine≥ 1, 5 mg/d. L (133µMol).

Conclusions ‒ The prosthetic valve adds a prothrombotic environment ‒ The TAVR population is also at high risk for embolism and bleeding ‒ Antithrombotic regimens after TAVR are expert consensusbased and influenced by patient comorbidities ‒ This calls for the evaluation of other anticoagulation regimens in RCT