TAVR With the Medtronic TAVR System In Patients








- Slides: 16

TAVR With the Medtronic TAVR System In Patients at Low Risk for Surgical Aortic Valve Replacement Jeffrey J. Popma, MD Professor of Medicine Harvard Medical School Director, Interventional Cardiology Beth Israel Deaconess Medical Center Boston, MA 1

Conflict of Interest Statement Over the past year, I have received the following: Institutional Grants: Medtronic, Boston Scientific, Abbott Vascular, Direct Flow, Cook Medical Advisory Board: Boston Scientific, Abbott Vascular, GE Healthcare, Covidien Non Vested Equity: Intelemage, Healthworks, Direct Flow Medical 2

Low Risk N=280 patients STS = 3. 0% Thyregod et al JACC 2015; 65: 2184– 94 3

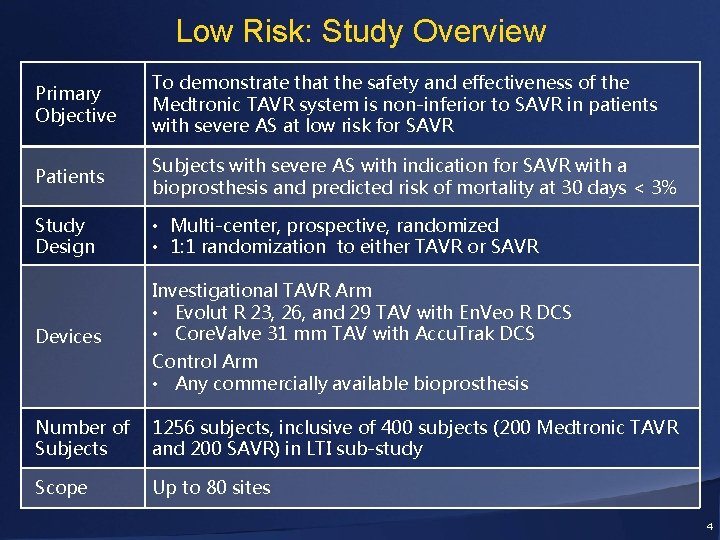
Low Risk: Study Overview Primary Objective To demonstrate that the safety and effectiveness of the Medtronic TAVR system is non-inferior to SAVR in patients with severe AS at low risk for SAVR Patients Subjects with severe AS with indication for SAVR with a bioprosthesis and predicted risk of mortality at 30 days < 3% Study Design • Multi-center, prospective, randomized • 1: 1 randomization to either TAVR or SAVR Devices Investigational TAVR Arm • Evolut R 23, 26, and 29 TAV with En. Veo R DCS • Core. Valve 31 mm TAV with Accu. Trak DCS Control Arm • Any commercially available bioprosthesis Number of Subjects 1256 subjects, inclusive of 400 subjects (200 Medtronic TAVR and 200 SAVR) in LTI sub-study Scope Up to 80 sites 4

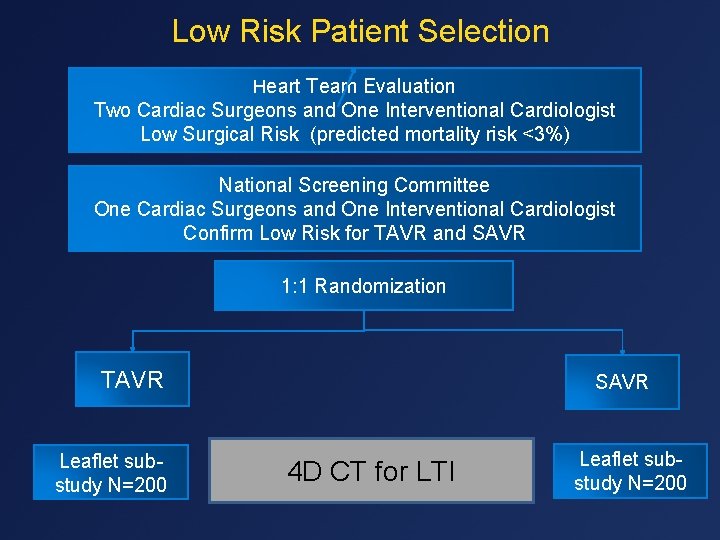
Low Risk Patient Selection Heart Team Evaluation Two Cardiac Surgeons and One Interventional Cardiologist Low Surgical Risk (predicted mortality risk <3%) National Screening Committee One Cardiac Surgeons and One Interventional Cardiologist Confirm Low Risk for TAVR and SAVR 1: 1 Randomization TAVR Leaflet substudy N=200 SAVR 4 D CT for LTI Leaflet substudy N=200 5

Low Risk: Study Administration Co-Principal Investigators • Jeffrey J. Popma • Micheal J. Reardon Executive Committee • Jeffrey J. Popma • Michael J. Reardon • G. Michael Deeb • Stephen Yakubov Screening Committee • Jeffrey J. Popma • Micheal J. Reardon • G. Michael Deeb • Stephen Yakubov • Thomas Gleason Echo Core Lab • Mayo Clinic (J Oh) Clinical Events Committee • Harvard Clinical Research Institute Data Safety Monitoring Committee • Harvard Clinical Research Institute Core Pathology Lab • CV Pathology (R Virmani) MDCT Core Lab (LTI Sub-study) • St. Paul’s Hospital (J Leipsic, P Blanke) 6

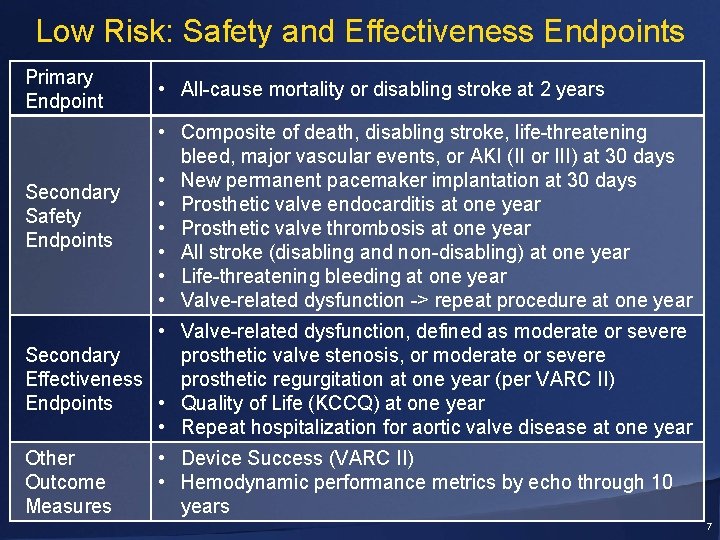
Low Risk: Safety and Effectiveness Endpoints Primary Endpoint • All-cause mortality or disabling stroke at 2 years Secondary Safety Endpoints • Composite of death, disabling stroke, life-threatening bleed, major vascular events, or AKI (II or III) at 30 days • New permanent pacemaker implantation at 30 days • Prosthetic valve endocarditis at one year • Prosthetic valve thrombosis at one year • All stroke (disabling and non-disabling) at one year • Life-threatening bleeding at one year • Valve-related dysfunction -> repeat procedure at one year • Valve-related dysfunction, defined as moderate or severe Secondary prosthetic valve stenosis, or moderate or severe Effectiveness prosthetic regurgitation at one year (per VARC II) Endpoints • Quality of Life (KCCQ) at one year • Repeat hospitalization for aortic valve disease at one year Other Outcome Measures • Device Success (VARC II) • Hemodynamic performance metrics by echo through 10 years 7

Low Risk Inclusion Criteria: What’s New? • < 3% heart team risk of mortality at 30 days • Symptomatic severe aortic stenosis, defined as AVA ≤ 1. 0 cm 2 (or AVAI ≤ 0. 6 cm 2/m 2), OR Mean gradient ≥ 40 mm. Hg, OR Max velocity ≥ 4. 0 m/s • Asymptomatic aortic stenosis – defined by an AVA ≤ 1. 0 cm 2 (or AVAI ≤ 0. 6 cm 2/m 2), AND max velocity ≥ 5. 0 m/s, OR AVA ≤ 1. 0 cm 2 (or AVAI ≤ 0. 6 cm 2/m 2), AND mean gradient ≥ 40 mm. Hg, OR Max velocity ≥ 4. 0 m/s AND positive ETT (limited exercise capacity, abnormal BP response, or arrhythmia) • Indicated for SAVR with bioprosthesis 8

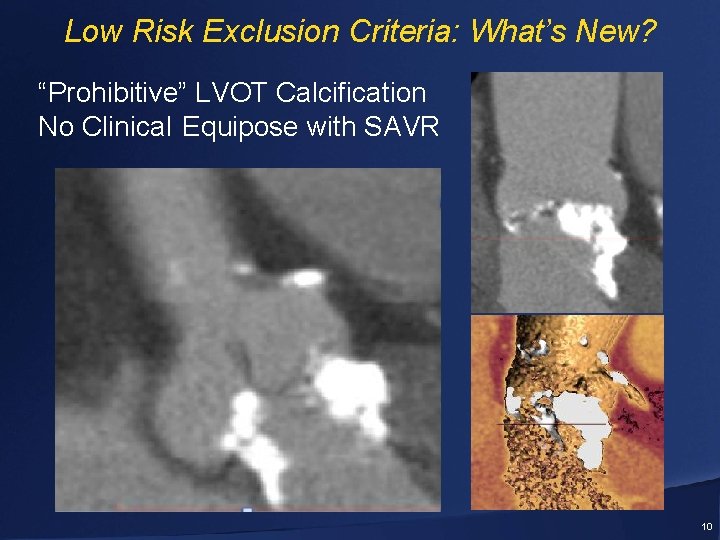
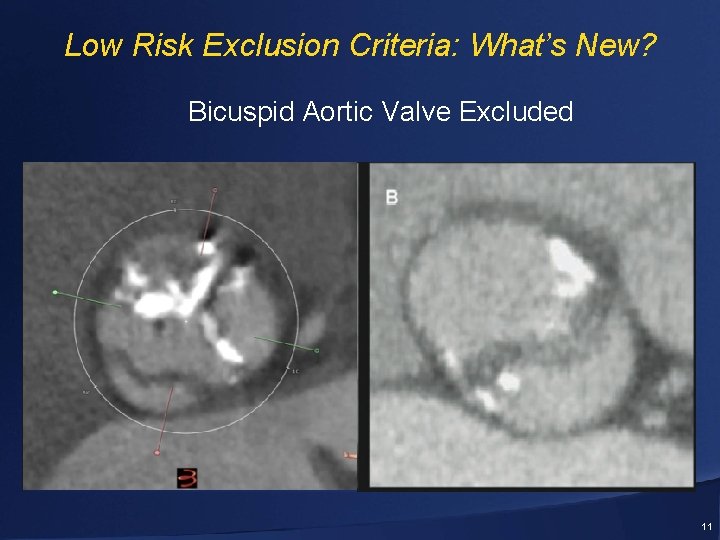
Low Risk: Exclusion Criteria: What’s New? • Bicuspid aortic valve • Multivessel CAD with Syntax score >22 and/or unprotected left main • Acute MI ≤ 30 days prior to the procedure • Severe MR or TR • Moderate or severe mitral stenosis • Aortic annulus < 18 mm or > 29 mm • Prohibitive LVOT calcification 9

Low Risk Exclusion Criteria: What’s New? “Prohibitive” LVOT Calcification No Clinical Equipose with SAVR 10

Low Risk Exclusion Criteria: What’s New? Bicuspid Aortic Valve Excluded 11

What is the Same with the US IDE Trials? Best Practices • CT based Sizing • Optimal Aortography • Post Procedural - Echo - Hemodynamics - Aortography • Post dilation for AR • Early Discharge First Patient Enrolled Pinnacle Health March 29, 2016 12

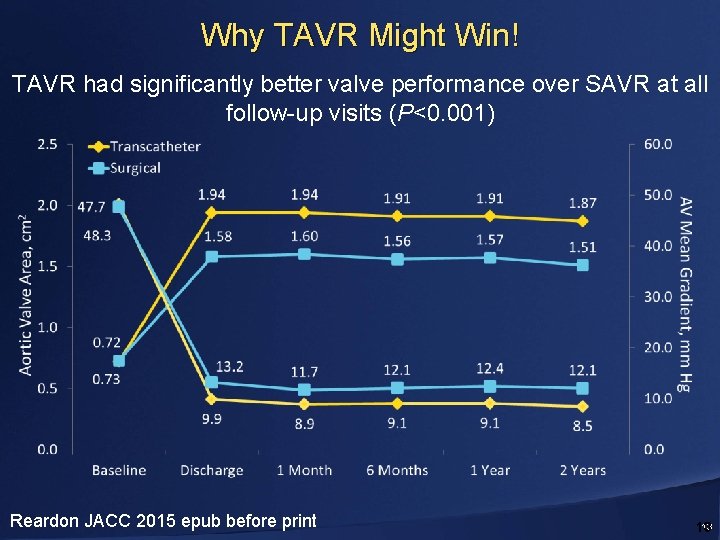
Why TAVR Might Win! TAVR had significantly better valve performance over SAVR at all follow-up visits (P<0. 001) Reardon JACC 2015 epub before print 13 13

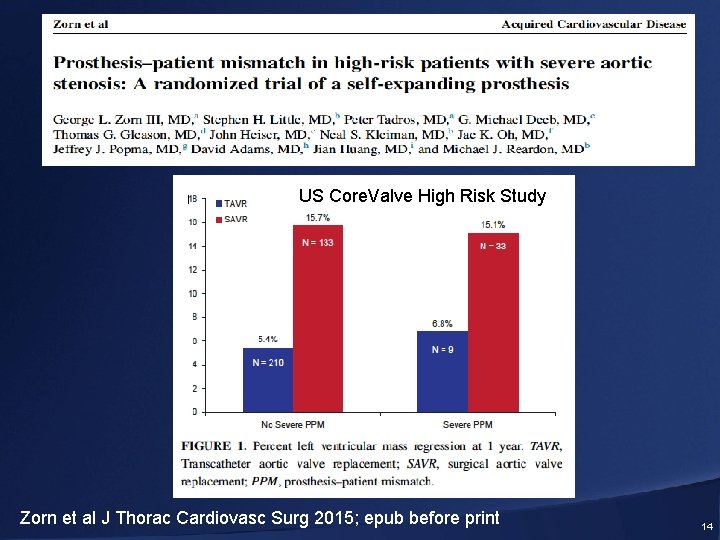
Severe PPM = EOAi < 0. 65 cm 2/m 2 US Core. Valve High Risk Study Zorn et al J Thorac Cardiovasc Surg 2015; epub before print 14

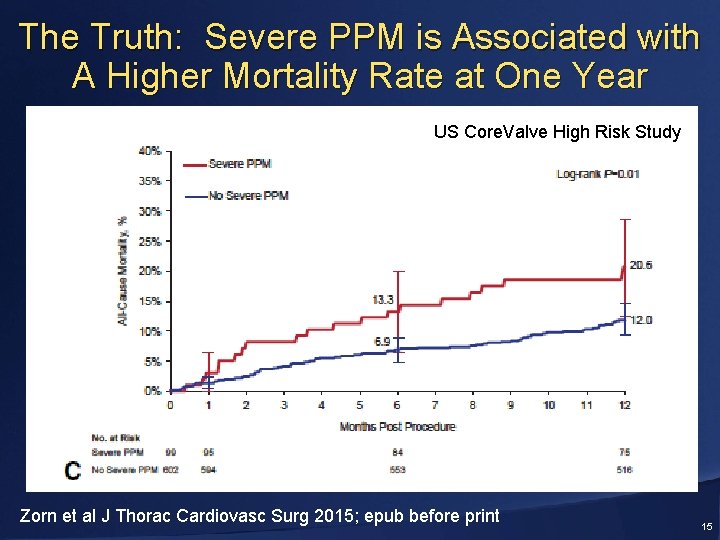
The Truth: Severe PPM is Associated with A Higher Mortality Rate at One Year US Core. Valve High Risk Study Zorn et al J Thorac Cardiovasc Surg 2015; epub before print 15

Summary A randomized trial is justified but it has to be meticulously performed with contemporary best practices and technically proficient surgeons and interventionalists. 16
 Annular rupture tavr
Annular rupture tavr Tavr antiplatelet guidelines
Tavr antiplatelet guidelines Medtronic purchasing portal
Medtronic purchasing portal Quality begins with me
Quality begins with me Vector express medtronic
Vector express medtronic Mpxr medtronic
Mpxr medtronic Insulin dose per kg
Insulin dose per kg Medtronic sofamor danek usa
Medtronic sofamor danek usa Branded icons
Branded icons Laura mauri
Laura mauri Medtronic structural heart
Medtronic structural heart Sextant medtronic
Sextant medtronic Medtronic pyramid
Medtronic pyramid Medtronic
Medtronic Nerven beckenbereich
Nerven beckenbereich Medtronic loop recorder lnq11
Medtronic loop recorder lnq11 Rachael scherer
Rachael scherer