CRT 2010 Washington DC January 21 2010 Medtronic






- Slides: 34

CRT 2010 Washington DC, January 21, 2010 Medtronic - Core Valve Device Evolution, Technique and Clinical Trial Update Eberhard Grube, MD, FACC, FSCAI St. Elisabeth Hospital, Heart Center Rhein-Ruhr, Essen, Germany Instituto Cardiologico Dante Pazzanese, São Paulo, Brazil

DISCLOSURES Eberhard Grube, MD Consulting Fees – Abbott Vascular, Boston Scientific Corporation, Cordis, a Johnson & Johnson Company, Medtronic Cardio. Vascular, Inc. Honoraria – Biosensors International , Boston Scientific Corporation, Medtronic Cardio. Vascular, Inc Ownership Interest (Stocks, Stock Options or Other Ownership Interest) – Biosensors International , Medtronic Cardio. Vascular, Inc. I intend to reference unlabeled/ unapproved uses of drugs or devices in my presentation. I intend to reference off-label use of stents and valve prosthesis.

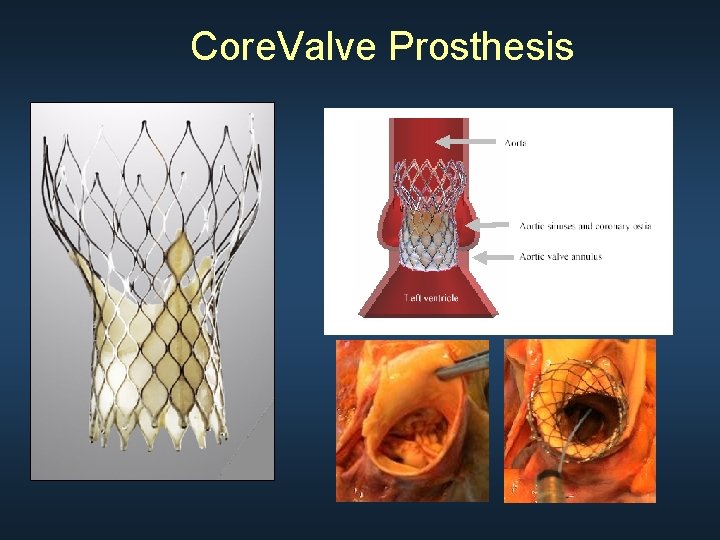
Core. Valve Prosthesis

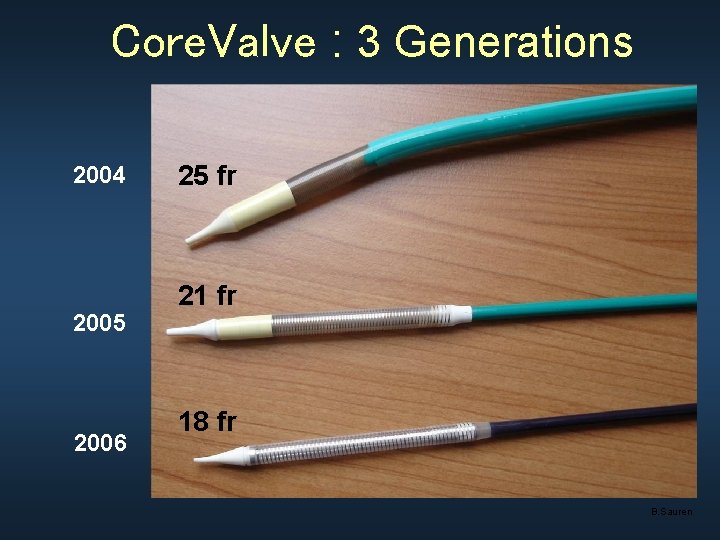
Core. Valve : 3 Generations 2004 2005 2006 25 fr 21 fr 18 fr B. Sauren

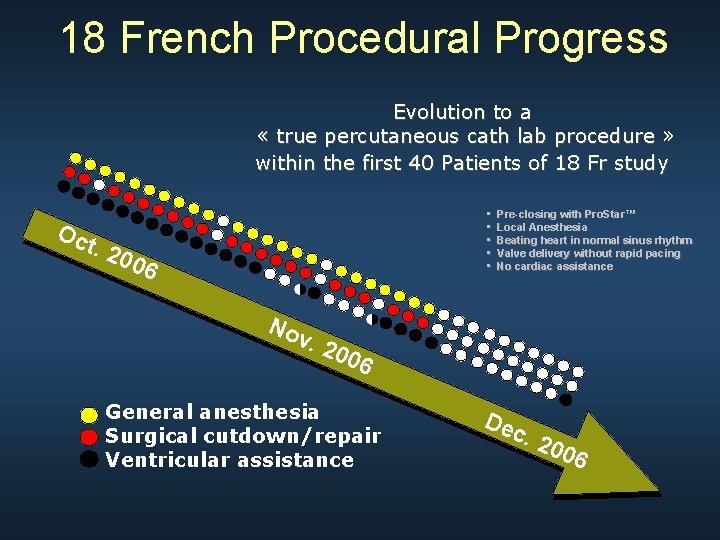
18 French Procedural Progress Evolution to a « true percutaneous cath lab procedure » within the first 40 Patients of 18 Fr study • • • Oc t. 2 006 No Pre-closing with Pro. Star™ Local Anesthesia Beating heart in normal sinus rhythm Valve delivery without rapid pacing No cardiac assistance v. 2 006 General anesthesia Surgical cutdown/repair Ventricular assistance Dec . 20 06

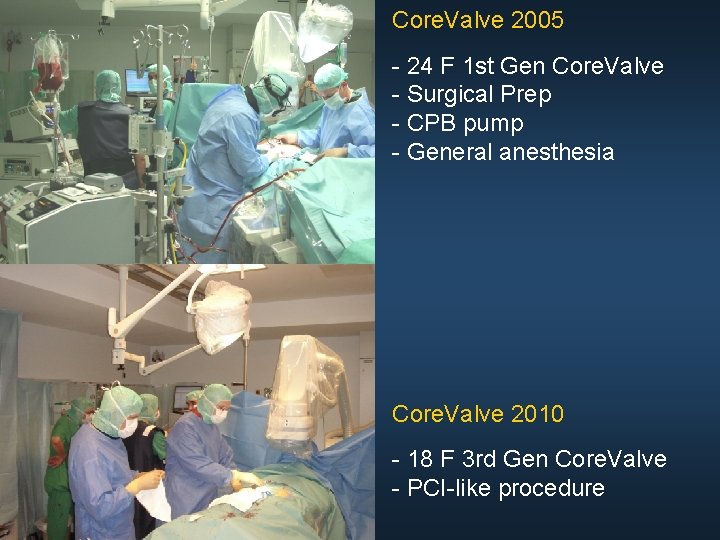
Core. Valve 2005 - 24 F 1 st Gen Core. Valve - Surgical Prep - CPB pump - General anesthesia Core. Valve 2010 - 18 F 3 rd Gen Core. Valve - PCI-like procedure

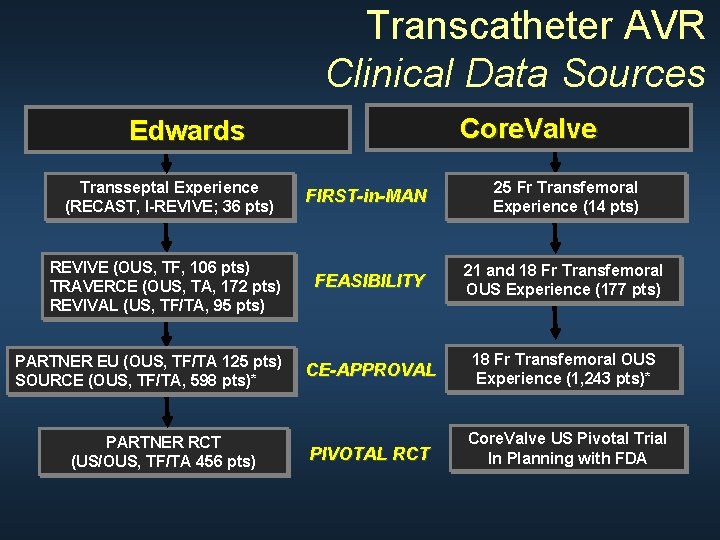
Transcatheter AVR Clinical Data Sources Core. Valve Edwards Transseptal Experience (RECAST, I-REVIVE; 36 pts) FIRST-in-MAN 25 Fr Transfemoral Experience (14 pts) REVIVE (OUS, TF, 106 pts) TRAVERCE (OUS, TA, 172 pts) REVIVAL (US, TF/TA, 95 pts) FEASIBILITY 21 and 18 Fr Transfemoral OUS Experience (177 pts) CE-APPROVAL 18 Fr Transfemoral OUS Experience (1, 243 pts)* PIVOTAL RCT Core. Valve US Pivotal Trial In Planning with FDA PARTNER EU (OUS, TF/TA 125 pts) SOURCE (OUS, TF/TA, 598 pts)* PARTNER RCT (US/OUS, TF/TA 456 pts)

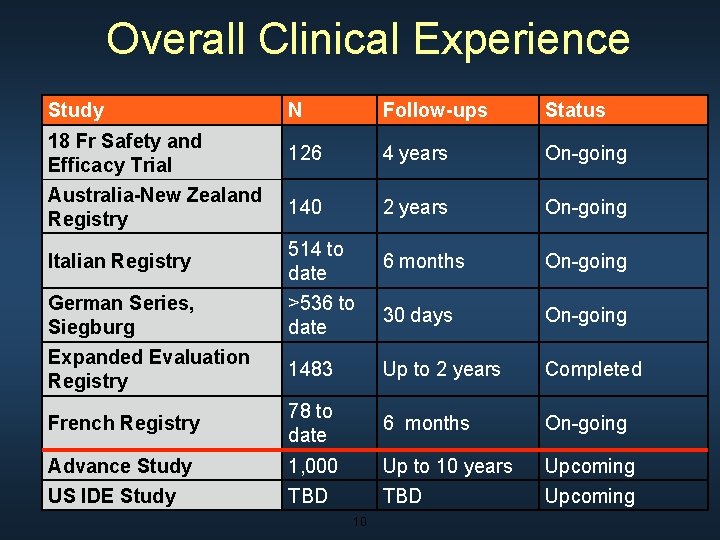
Overall Clinical Experience Study N Follow-ups Status 18 Fr Safety and Efficacy Trial 126 4 years On-going Australia-New Zealand Registry 140 2 years On-going Italian Registry 514 to date 6 months On-going German Series, Siegburg >536 to date 30 days On-going Expanded Evaluation Registry 1483 Up to 2 years Completed French Registry 78 to date 6 months On-going Advance Study 1, 000 Up to 10 years Upcoming US IDE Study TBD Upcoming 10

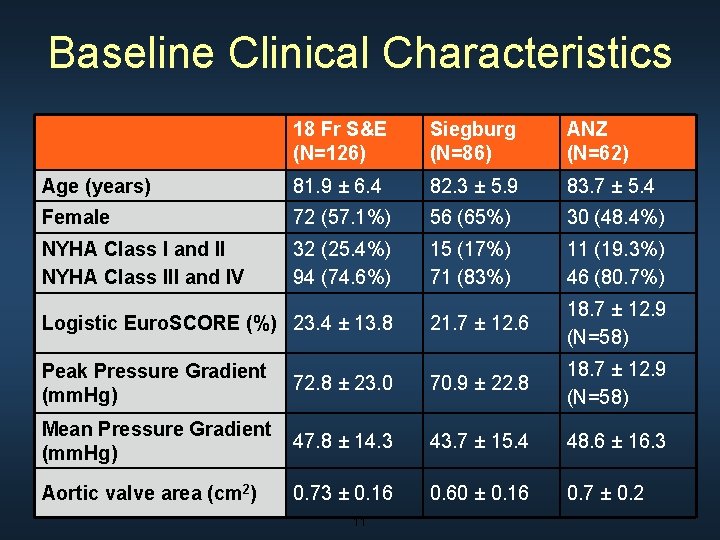
Baseline Clinical Characteristics 18 Fr S&E (N=126) Siegburg (N=86) ANZ (N=62) Age (years) 81. 9 ± 6. 4 82. 3 ± 5. 9 83. 7 ± 5. 4 Female 72 (57. 1%) 56 (65%) 30 (48. 4%) NYHA Class I and II NYHA Class III and IV 32 (25. 4%) 94 (74. 6%) 15 (17%) 71 (83%) 11 (19. 3%) 46 (80. 7%) 21. 7 ± 12. 6 18. 7 ± 12. 9 (N=58) Logistic Euro. SCORE (%) 23. 4 ± 13. 8 Peak Pressure Gradient (mm. Hg) 72. 8 ± 23. 0 70. 9 ± 22. 8 18. 7 ± 12. 9 (N=58) Mean Pressure Gradient (mm. Hg) 47. 8 ± 14. 3 43. 7 ± 15. 4 48. 6 ± 16. 3 Aortic valve area (cm 2) 0. 73 ± 0. 16 0. 60 ± 0. 16 0. 7 ± 0. 2 11

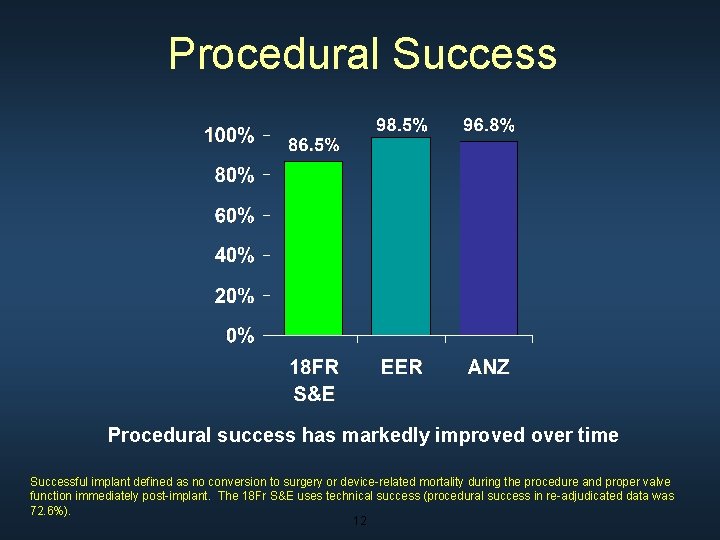
Procedural Success Procedural success has markedly improved over time Successful implant defined as no conversion to surgery or device-related mortality during the procedure and proper valve function immediately post-implant. The 18 Fr S&E uses technical success (procedural success in re-adjudicated data was 72. 6%). 12

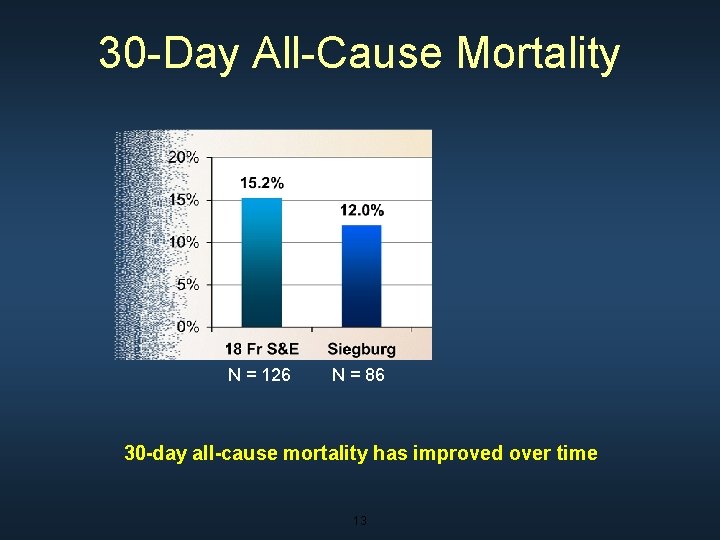
30 -Day All-Cause Mortality N = 126 N = 86 30 -day all-cause mortality has improved over time 13

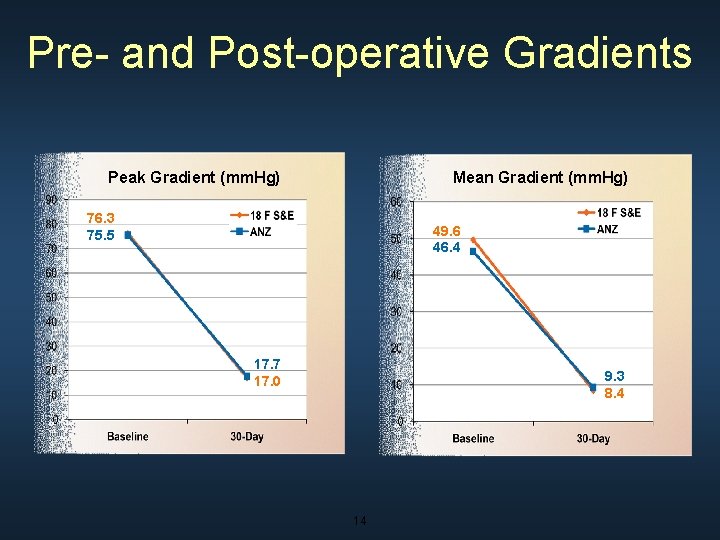
Pre- and Post-operative Gradients Peak Gradient (mm. Hg) Mean Gradient (mm. Hg) 76. 3 75. 5 49. 6 46. 4 17. 7 17. 0 9. 3 8. 4 14

Change in NYHA Class Paired 30 -Day NYHA Classification 15

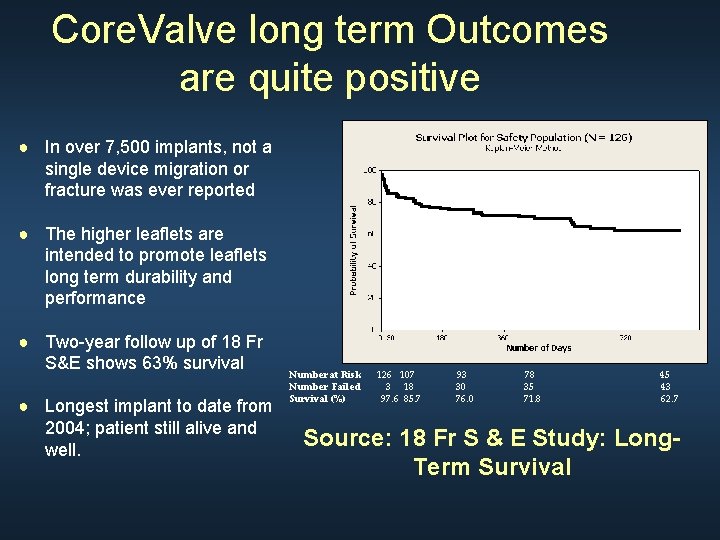
Core. Valve long term Outcomes are quite positive ● In over 7, 500 implants, not a single device migration or fracture was ever reported ● The higher leaflets are intended to promote leaflets long term durability and performance ● Two-year follow up of 18 Fr S&E shows 63% survival ● Longest implant to date from 2004; patient still alive and well. Number at Risk Number Failed Survival (%) 126 107 3 18 97. 6 85. 7 93 30 76. 0 78 35 71. 8 45 43 62. 7 Source: 18 Fr S & E Study: Long. Term Survival

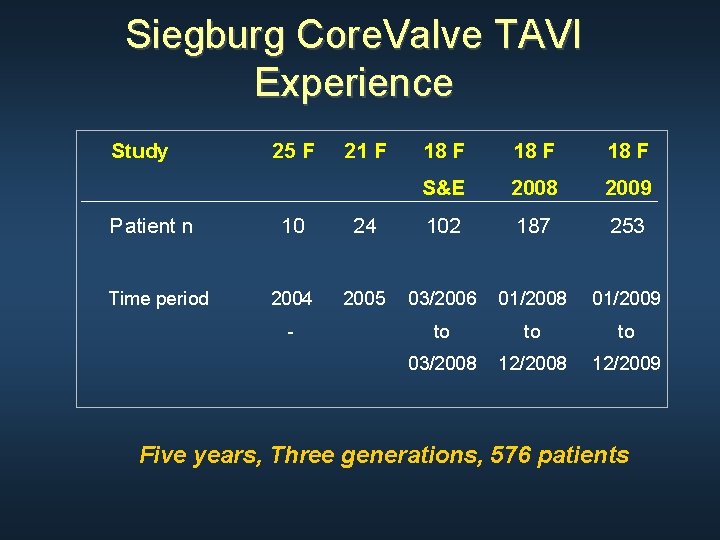
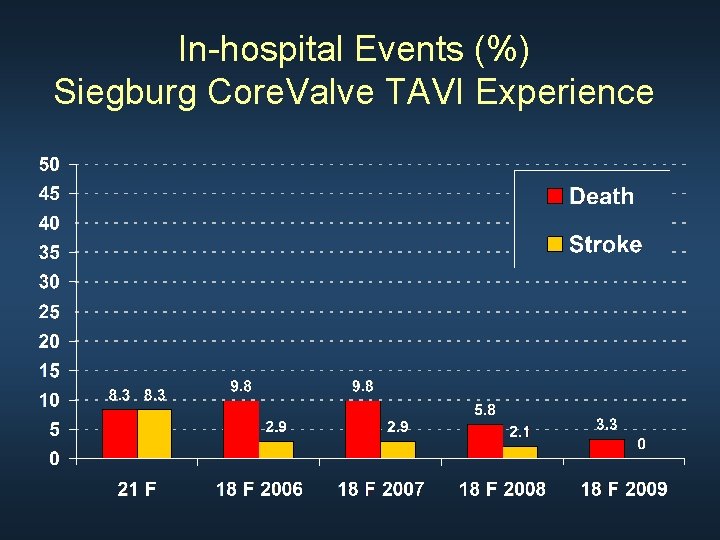
Siegburg Core. Valve TAVI Experience Study Patient n Time period 25 F 21 F 18 F S&E 2008 2009 10 24 102 187 253 2004 2005 03/2006 01/2008 01/2009 to to to 03/2008 12/2009 - Five years, Three generations, 576 patients

In-hospital Events (%) Siegburg Core. Valve TAVI Experience

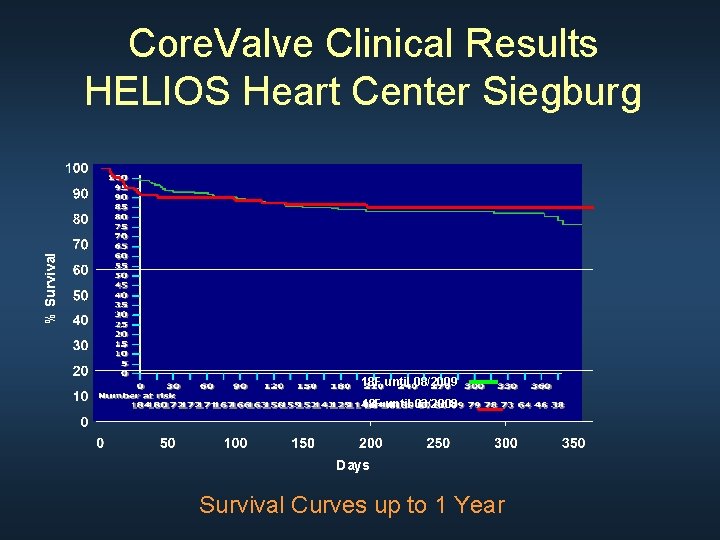
% Survival Core. Valve Clinical Results HELIOS Heart Center Siegburg 18 F until 08/2009 18 F until 03/2008 Days Survival Curves up to 1 Year

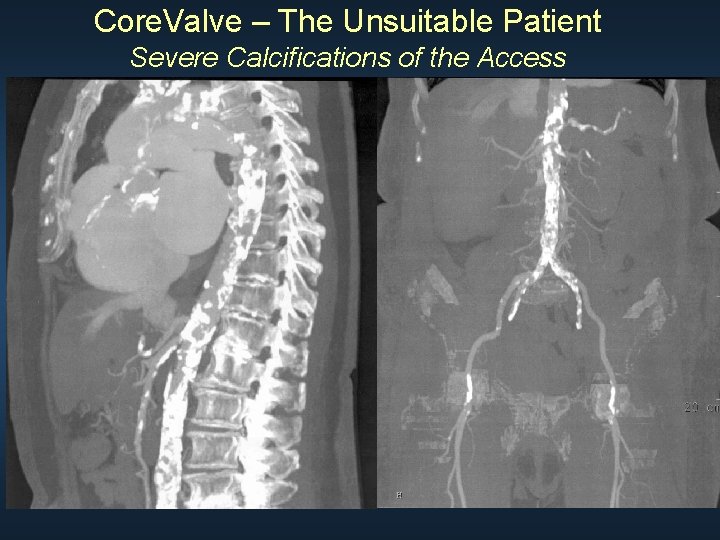
Core. Valve – The Unsuitable Patient Severe Calcifications of the Access

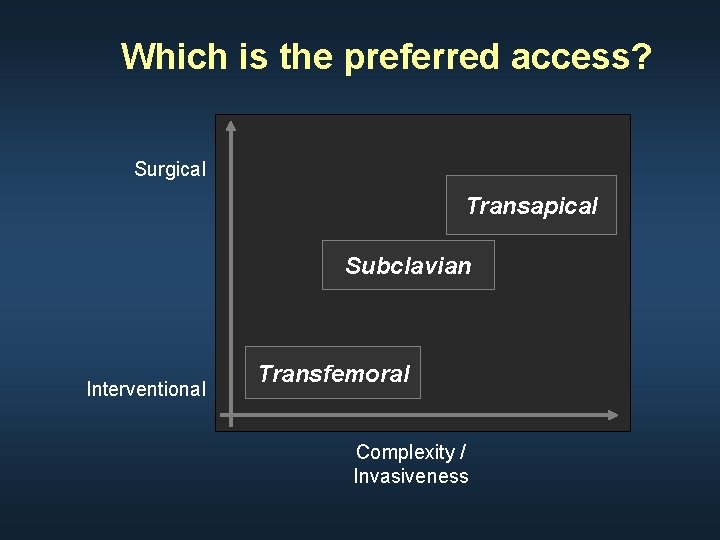
Which is the preferred access? Surgical Transapical Subclavian Interventional Transfemoral Complexity / Invasiveness

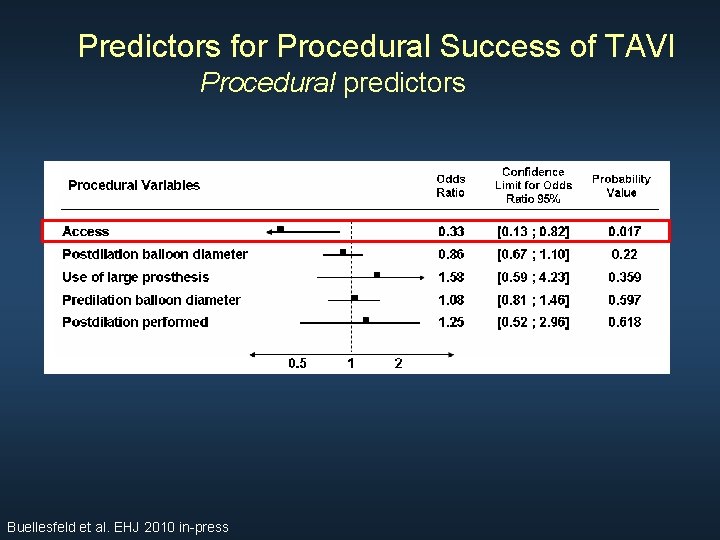
Predictors for Procedural Success of TAVI Procedural predictors Buellesfeld et al. EHJ 2010 in-press

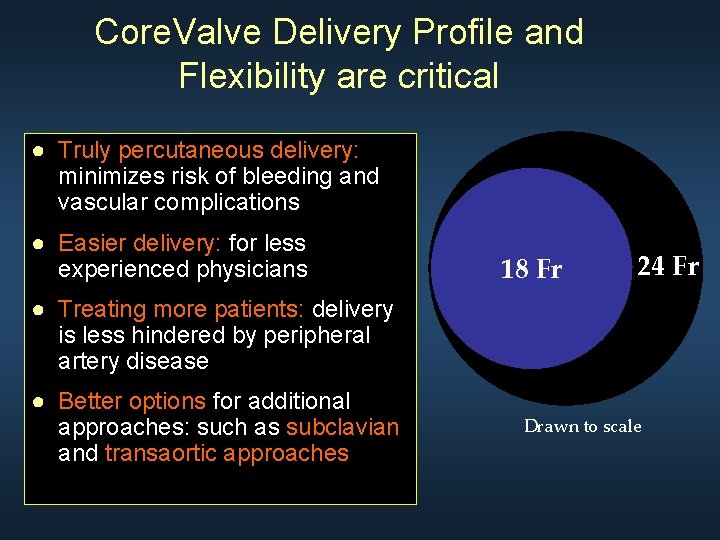
Core. Valve Delivery Profile and Flexibility are critical ● Truly percutaneous delivery: minimizes risk of bleeding and vascular complications ● Easier delivery: for less experienced physicians 18 Fr 24 Fr ● Treating more patients: delivery is less hindered by peripheral artery disease ● Better options for additional approaches: such as subclavian and transaortic approaches Drawn to scale

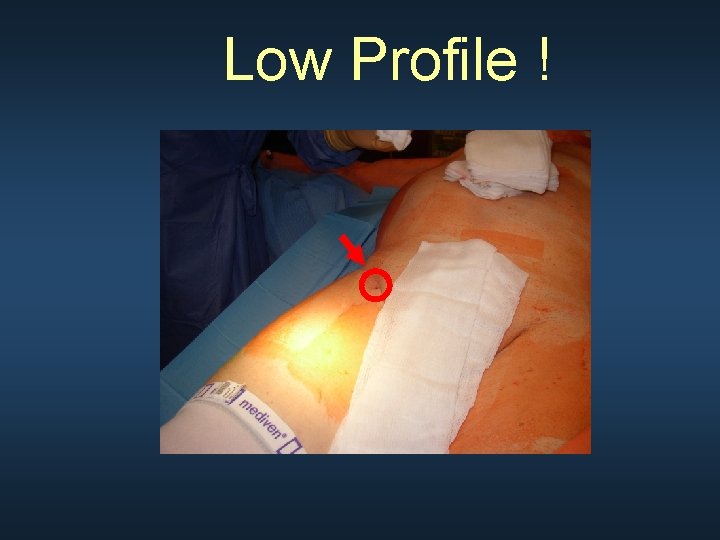
Low Profile !

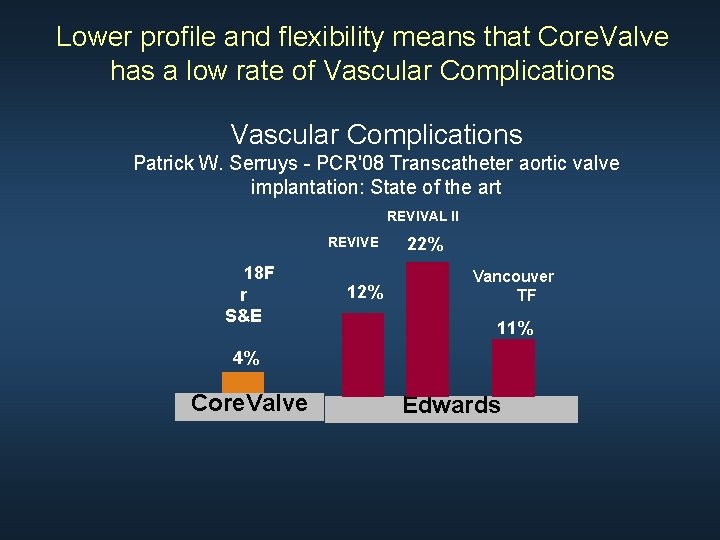
Lower profile and flexibility means that Core. Valve has a low rate of Vascular Complications Patrick W. Serruys - PCR'08 Transcatheter aortic valve implantation: State of the art REVIVAL II REVIVE 18 F r S&E 12% 22% Vancouver TF 11% 4% Core. Valve Edwards

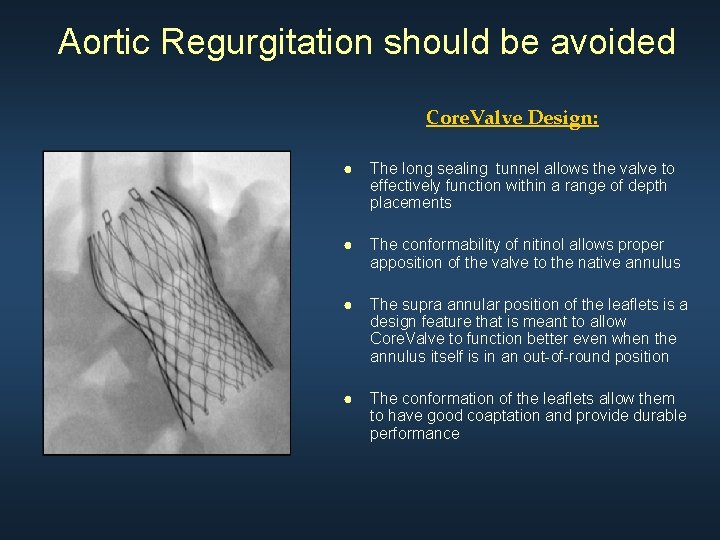
Aortic Regurgitation should be avoided Core. Valve Design: ● The long sealing tunnel allows the valve to effectively function within a range of depth placements ● The conformability of nitinol allows proper apposition of the valve to the native annulus ● The supra annular position of the leaflets is a design feature that is meant to allow Core. Valve to function better even when the annulus itself is in an out-of-round position ● The conformation of the leaflets allow them to have good coaptation and provide durable performance

Core. Valve is functioning well in Out-of. Round Situations ● Core. Valve has been shown to retain a round mid-section (where the leaflets are), even when the annulus was out of round - “Dual source MSCT demonstrated incomplete and non-uniform expansion of the CRS frame, but the functionally important mid-segment was well expanded and almost symmetrical. Anatomical under sizing and incomplete apposition of struts was seen in the majority of patients. ” (09/09) Schultz CJ, Weustink A, Piazza N, Otten A, Mollet N, Krestin G, van Geuns RJ, de Feyter P, Serruys PW, de Jaegere P. Geometry and degree of apposition of the Core. Valve Re. Valving system with multislice computed tomography after implantation in patients with aortic stenosis. J Am Coll Cardiol 2009; 54(10): 911 -918

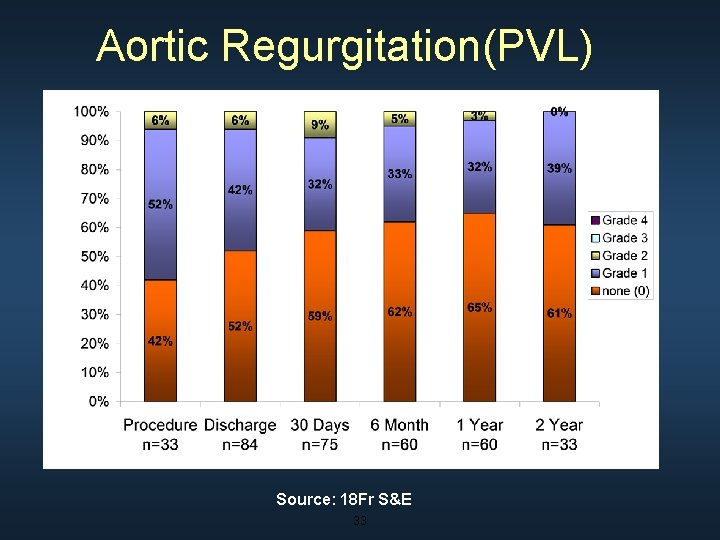
Aortic Regurgitation(PVL) Source: 18 Fr S&E 33

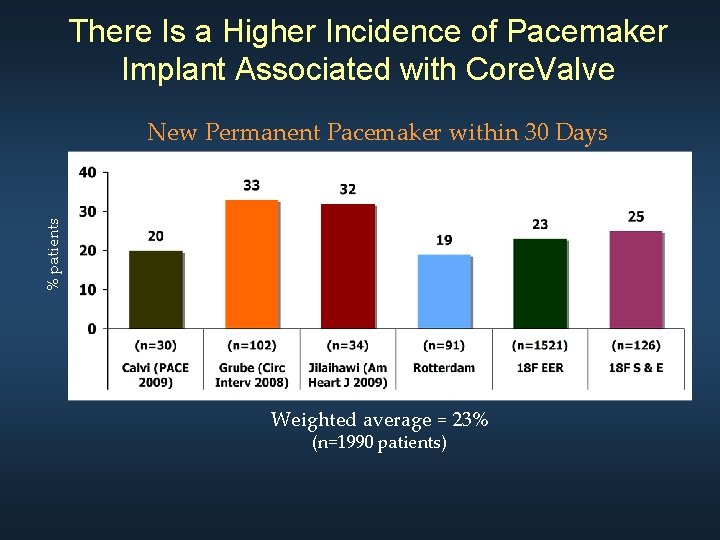
There Is a Higher Incidence of Pacemaker Implant Associated with Core. Valve % patients New Permanent Pacemaker within 30 Days Weighted average = 23% (n=1990 patients)

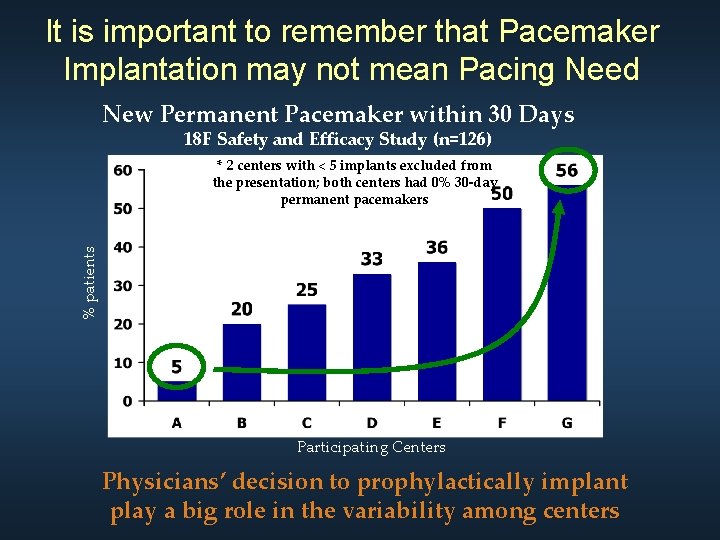
It is important to remember that Pacemaker Implantation may not mean Pacing Need New Permanent Pacemaker within 30 Days 18 F Safety and Efficacy Study (n=126) % patients * 2 centers with < 5 implants excluded from the presentation; both centers had 0% 30 -day permanent pacemakers Participating Centers Physicians’ decision to prophylactically implant play a big role in the variability among centers

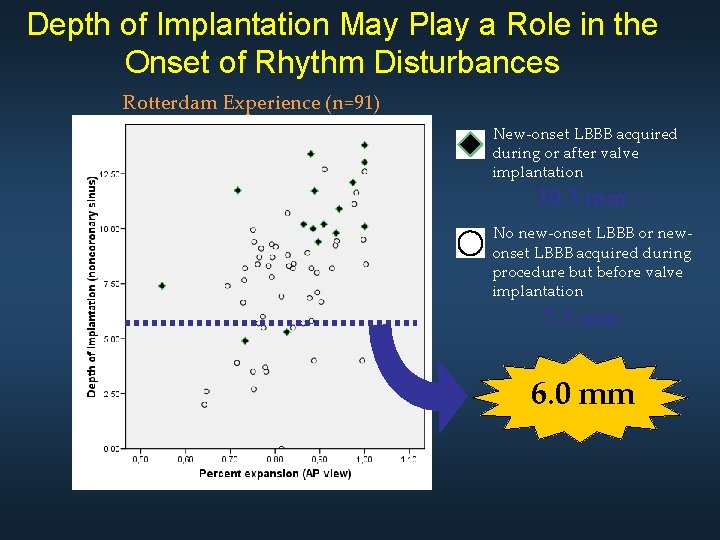
Depth of Implantation May Play a Role in the Onset of Rhythm Disturbances Rotterdam Experience (n=91) New-onset LBBB acquired during or after valve implantation 10. 3 mm No new-onset LBBB or newonset LBBB acquired during procedure but before valve implantation 7. 3 mm 6. 0 mm

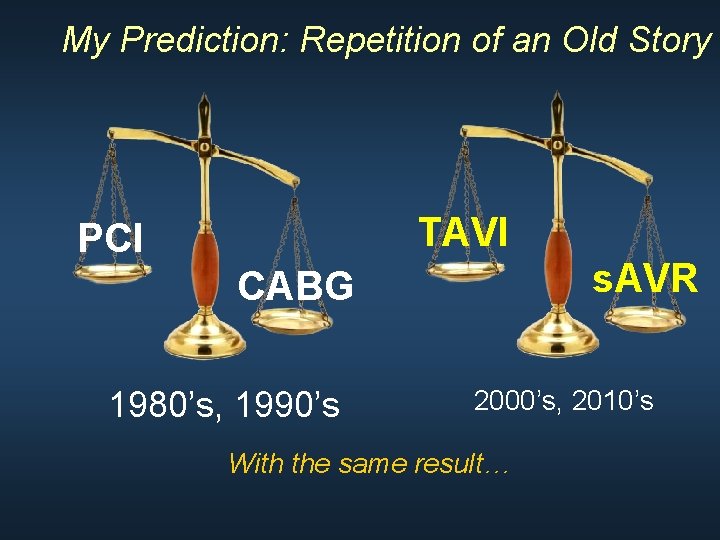
My Prediction: Repetition of an Old Story TAVI PCI s. AVR CABG 1980’s, 1990’s 2000’s, 2010’s With the same result…

Thank you for your attention !

Questions ?

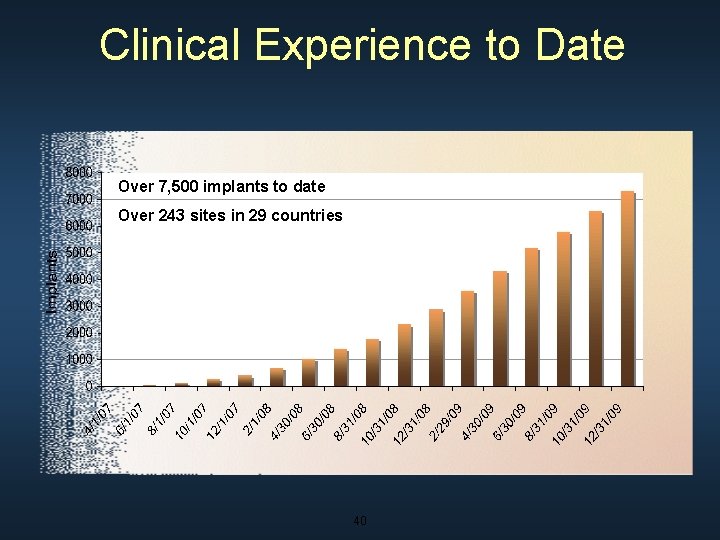
Clinical Experience to Date Over 7, 500 implants to date Over 243 sites in 29 countries 40

Thank you for your attention !
 Crt 2010
Crt 2010 Medtronic
Medtronic Progenix dbm
Progenix dbm Medtronic minimed 530g
Medtronic minimed 530g Rick kuntz
Rick kuntz Medtronic structural heart
Medtronic structural heart Mpxr medtronic
Mpxr medtronic Quality begin with me
Quality begin with me Nerven beckenbereich
Nerven beckenbereich Medtronic sofamor danek usa
Medtronic sofamor danek usa Medtronic project zeus
Medtronic project zeus Sean salmon medtronic salary
Sean salmon medtronic salary Sextant medtronic
Sextant medtronic Medtronic harmony
Medtronic harmony Lvcm medtronic
Lvcm medtronic Rachael scherer
Rachael scherer Reveal linq app
Reveal linq app Brandcentral medtronic
Brandcentral medtronic Biletec
Biletec Medtronic tlif
Medtronic tlif Medtronic sofamor danek usa
Medtronic sofamor danek usa Rick kuntz
Rick kuntz Mpxr medtronic
Mpxr medtronic John wainwright medtronic
John wainwright medtronic Laura mauri md
Laura mauri md Medtronic symplicity
Medtronic symplicity Medtronic supplier change portal
Medtronic supplier change portal Crt monitor vorteile nachteile
Crt monitor vorteile nachteile Display devices in computer graphics
Display devices in computer graphics Digital cockpit display unit
Digital cockpit display unit Monitor crt partes
Monitor crt partes Uses crt;
Uses crt; Rsa crt calculator
Rsa crt calculator Uses crt; var
Uses crt; var Crt 2017
Crt 2017