Aim What is entropy Spontaneous Reaction A spontaneous








- Slides: 12

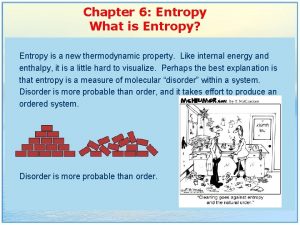
Aim: What is entropy?

Spontaneous Reaction • A spontaneous reaction is one that is able to proceed without needing an outside source of energy • A nonspontaneous reaction needs an input of energy.

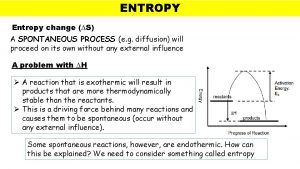
Spontaneous reactions occur in the direction of: 1. Less energy (lower enthalpy): exothermic reactions are favored 2. Greater entropy (randomness, disorder)

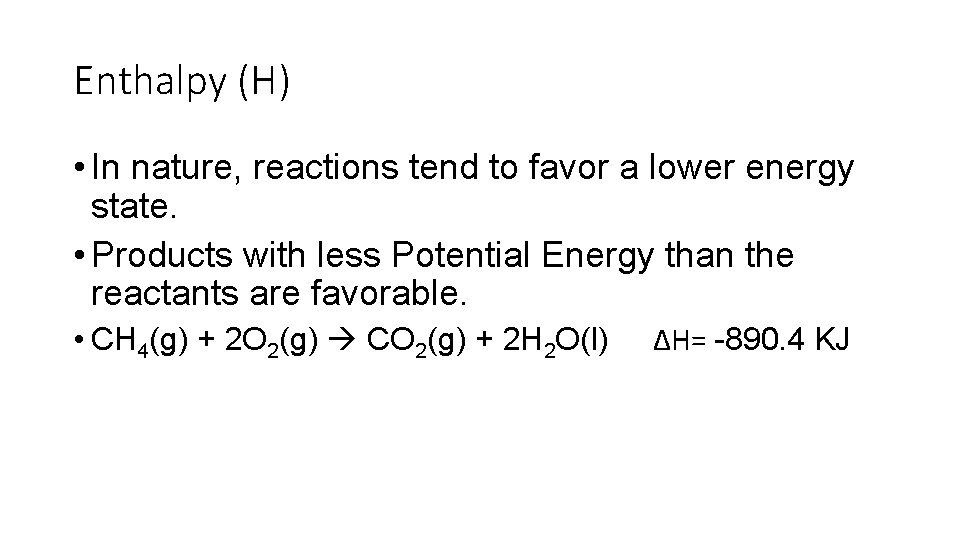
Enthalpy (H) • In nature, reactions tend to favor a lower energy state. • Products with less Potential Energy than the reactants are favorable. • CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) ΔH= -890. 4 KJ

Entropy

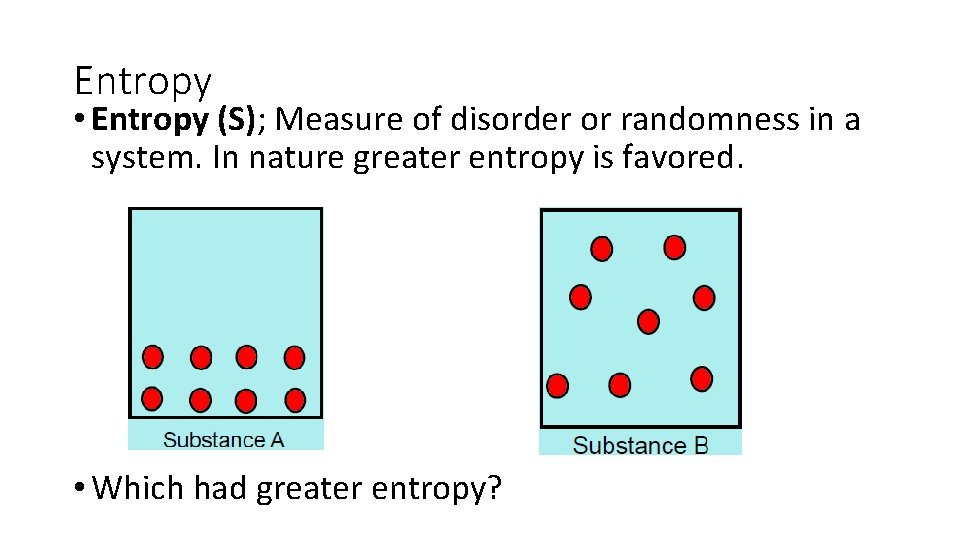
Entropy • Entropy (S); Measure of disorder or randomness in a system. In nature greater entropy is favored. • Which had greater entropy?

Entropy 1. Which phase will have the greatest entropy? The least entropy?

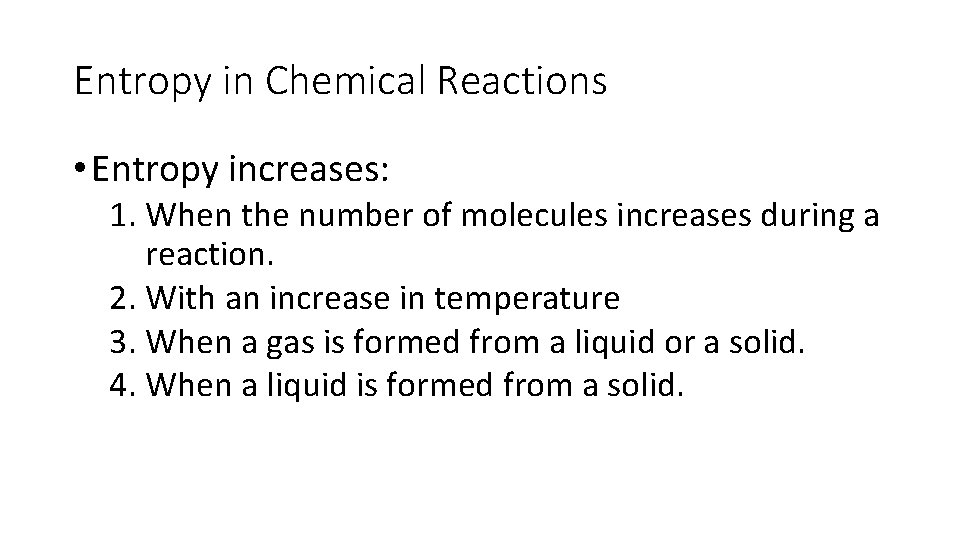
Entropy in Chemical Reactions • Entropy increases: 1. When the number of molecules increases during a reaction. 2. With an increase in temperature 3. When a gas is formed from a liquid or a solid. 4. When a liquid is formed from a solid.

Questions 1. Which tendencies favor a spontaneous reaction? 1) lower energy and decreasing entropy 2) lower energy and increasing entropy 3) higher energy and decreasing entropy 4) higher energy and increasing entropy

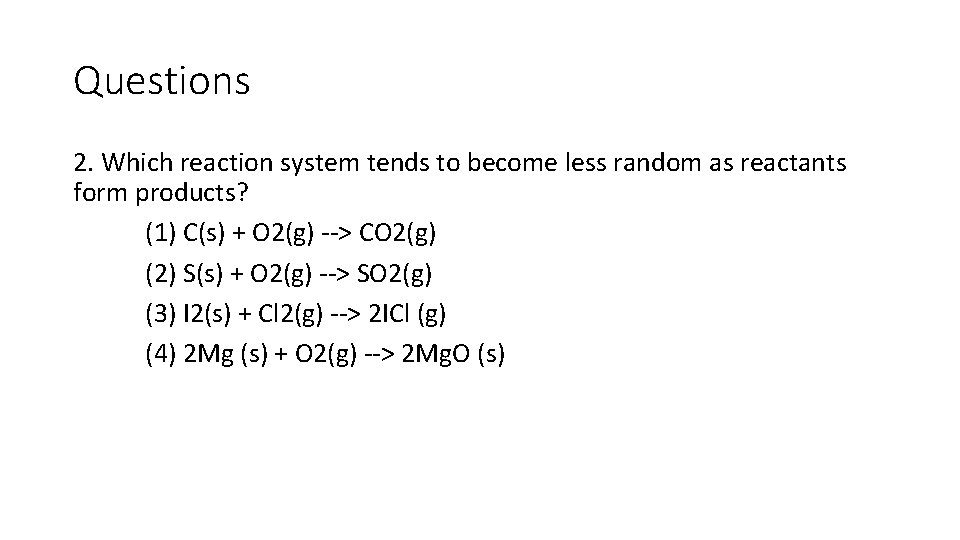
Questions 2. Which reaction system tends to become less random as reactants form products? (1) C(s) + O 2(g) --> CO 2(g) (2) S(s) + O 2(g) --> SO 2(g) (3) I 2(s) + Cl 2(g) --> 2 ICl (g) (4) 2 Mg (s) + O 2(g) --> 2 Mg. O (s)

Questions 3. As Na. Cl(s) dissolves according to the equation Na. Cl(s) --> Na+ (aq) + Cl- (aq) the entropy of the system (1) decreases (2) increases (3) remains the same

 Spontaneous reaction
Spontaneous reaction Concept of free energy
Concept of free energy Electrolysis spontaneous or nonspontaneous
Electrolysis spontaneous or nonspontaneous Which tendencies favor a spontaneous reaction
Which tendencies favor a spontaneous reaction Spontaneous reaction chemistry
Spontaneous reaction chemistry Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Activity formula
Activity formula Order of reaction
Order of reaction Ictahedron
Ictahedron Non spontaneous process
Non spontaneous process Entropy in thermodynamics
Entropy in thermodynamics Entropy change formula
Entropy change formula