Adjuvant Therapy in Melanoma Dr Tania Tillett Medical






- Slides: 19

Adjuvant Therapy in Melanoma Dr Tania Tillett – Medical Oncology Consultant

Introduction l l l Background Evidence Current Practice Cancer Drugs Fund Rules Questions

Background l Curative – Stage III n n l Palliative – Stage IV n l Neoadjuvant (drugs before surgery) Adjuvant (drugs after surgery) – 1 year of drugs Drugs and/or surgery – drugs until toxicity or progression Malignant melanoma n n 40% BRAF positive – can use tablets (dabrafenib and trametinib) or immunotherapy 60% BRAF wild-type – can only use immunotherapy

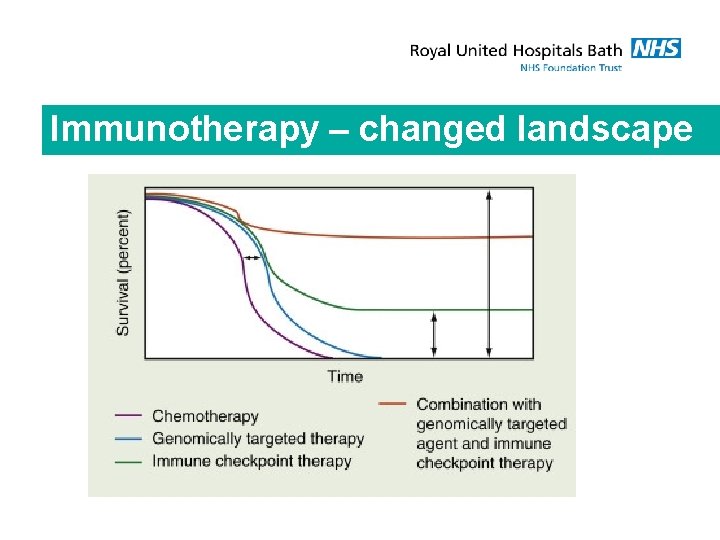
Immunotherapy – changed landscape

Evidence for adjuvant therapy l l Check. Mate 238 Phase III RCT n=906 Randomised 1 year of Nivo or Ipi Eligability n n Resected Stage IIIB or IIIC or IV within 12 weeks of resection Occular excluded Acral and mucosal allowed Patients on steroids or with auto-immune conditions excluded

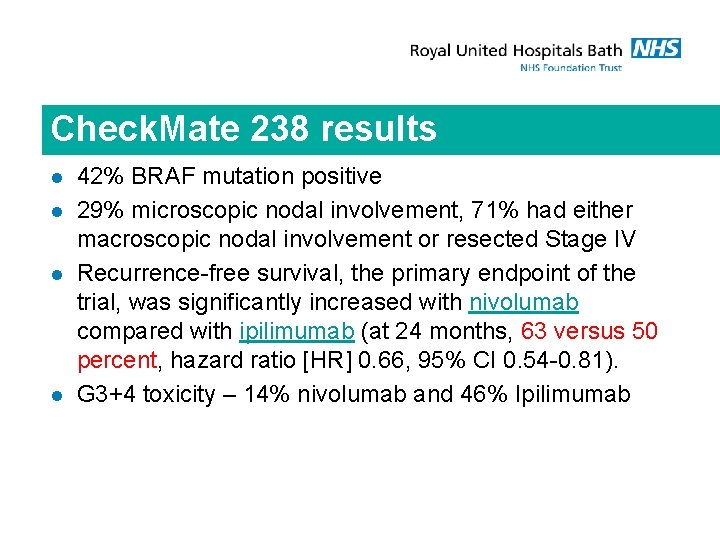
Check. Mate 238 results l l 42% BRAF mutation positive 29% microscopic nodal involvement, 71% had either macroscopic nodal involvement or resected Stage IV Recurrence-free survival, the primary endpoint of the trial, was significantly increased with nivolumab compared with ipilimumab (at 24 months, 63 versus 50 percent, hazard ratio [HR] 0. 66, 95% CI 0. 54 -0. 81). G 3+4 toxicity – 14% nivolumab and 46% Ipilimumab

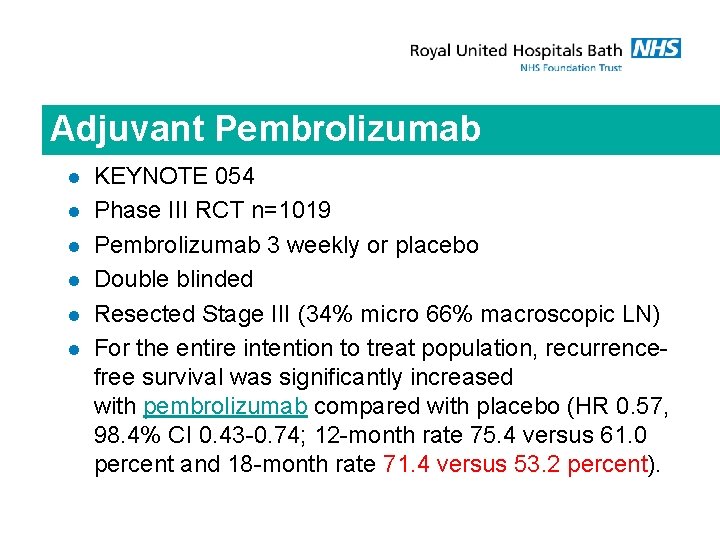
Adjuvant Pembrolizumab l l l KEYNOTE 054 Phase III RCT n=1019 Pembrolizumab 3 weekly or placebo Double blinded Resected Stage III (34% micro 66% macroscopic LN) For the entire intention to treat population, recurrencefree survival was significantly increased with pembrolizumab compared with placebo (HR 0. 57, 98. 4% CI 0. 43 -0. 74; 12 -month rate 75. 4 versus 61. 0 percent and 18 -month rate 71. 4 versus 53. 2 percent).


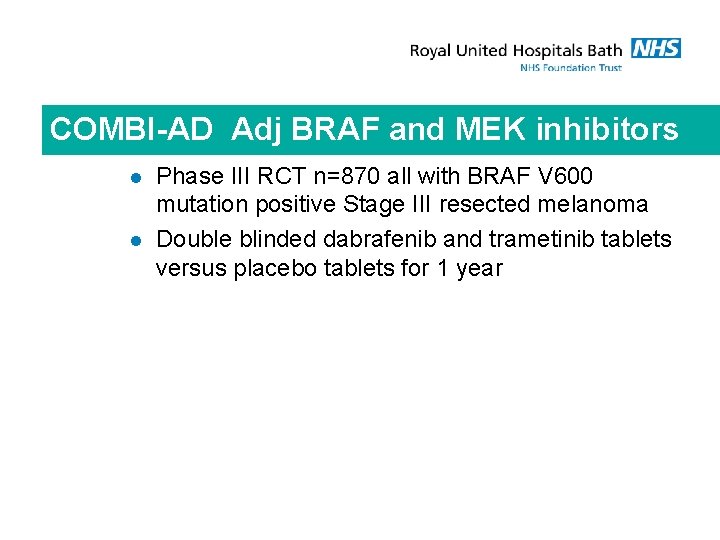
COMBI-AD Adj BRAF and MEK inhibitors l l Phase III RCT n=870 all with BRAF V 600 mutation positive Stage III resected melanoma Double blinded dabrafenib and trametinib tablets versus placebo tablets for 1 year

COMBI AD results – median fu 3. 7 yrs l l Relapse-free survival, the primary endpoint of the trial, was longer with dabrafenib plus trametinib compared with placebo (three-year rate, 59 versus 40 percent; four-year rate, 54 versus 38 percent; hazard ratio [HR] 0. 49, 95% CI 0. 40 -0. 59), with treatment benefits observed irrespective of baseline factors, according to subgroup analysis [15]. Overall survival, at a median follow-up of 2. 8 years, was prolonged with the targeted therapy (three-year rate, 86 versus 77 percent; HR 0. 57, 95% CI 0. 42 -0. 79) [6], but remains immature. The estimated cure rate at a median follow-up of 3. 5 years was 54 (95% CI 49 -59) percent in the dabrafenib plus trametinib arm versus 37 (95% CI 32 -42) percent in the placebo arm.

Current Practice l Adjuvant patients must BE DRUG NIAVE Stage III resected n n l BRAF positive – either 1 year of dabrafenib+trametinib tablets, or 1 year of 3 weekly Pembrolizumab or 1 year of 2 weekly Nivolumab BRAF negative - 1 year of 3 weekly Pembrolizumab or 1 year of 2 weekly Nivolumab Stage IV resected n BRAF positive or negative – only options 1 year of 2 weekly Nivolumab

Guidance – Cancer Drugs Fund l Nivolumab for the adjuvant treatment of newly diagnosed and completely resected stage III or completely resected stage IV malignant melanoma where the following criteria are met: n 4. The patient has melanoma which has been staged according to the AJCC 8 th edition as stage III disease or completely resected stage IV disease. Please state which stage disease the patient has: Stage IIIA disease or Stage IIIB disease or Stage IIIC disease or Stage IIID disease or Stage IV disease that has been completely resected 5. If stage III melanoma, the disease has been completely resected via sentinel node biopsy (‘sentinel lymphadenectomy’) or when indicated via completion lymph node dissection and/or there has been complete resection of intransit metastases; if stage IV melanoma, the distant metastatic disease has been completely resected

CDF Continued l 8. The prescribing clinician has discussed with the patient the benefits and toxicities of adjuvant nivolumab in stage III or completely resected stage IV disease and if stage III disease, has used the expected median figures below in relation to the risk of disease relapse if a routine surveillance policy is followed: - for stage IIIA disease, the 5 and 10 year melanoma-specific survival probabilities with routine surveillance are 93% and 88%, respectively - for stage IIIB disease, the 5 and 10 year figures are 83% and 77%, respectively - for stage IIIC disease, the 5 and 10 year figures are 69% and 60%, respectively - for stage IIID disease, the 5 and 10 year figures are 32% and 24%, respectively

CDF Cont l 10. Treatment with nivolumab will be continued for a maximum of 12 months (or a maximum of 26 cycles if given 2 -weekly) from the start of treatment in the absence of disease recurrence, unacceptable toxicity or withdrawal of patient consent

Pembrolizumab adjuvantly l 4. The patient has melanoma which has been staged as stage III disease according to the AJCC 8 th edition. Please state which stage disease the patient has: Stage IIIA disease or Stage IIIB disease or Stage IIIC disease or Stage IIID disease 5. This stage III disease has been completely resected via sentinel node biopsy (‘sentinel lymphadenectomy’) or when indicated via completion lymph node dissection and/or there has been complete resection of intransit metastases.

Dabrafenib and Trametinib l Evidence-based recommendations on dabrafenib (Tafinlar) with trametinib (Mekinist) for resected stage III, BRAF V 600 mutation-positive melanoma in adults.

Questions l Are mucosal and ocular melanomas allowed? n l Why 12 weeks from surgery? n l CDF does not specify but trial only included mucosal CDF does not specify but this was a trial entry criteria and has biological plausibility Does it have to be first presentation? n The trials were but again biological plausibility but outside trial evidence


 Dr tania tillett
Dr tania tillett Adjuvant nsclc
Adjuvant nsclc Gondor adjuvant
Gondor adjuvant Domenico galetta
Domenico galetta Adjuvant neoadjuvant palliative
Adjuvant neoadjuvant palliative Melanoma causes
Melanoma causes Melanoma coroide metastasi fegato
Melanoma coroide metastasi fegato Atypical mole vs melanoma
Atypical mole vs melanoma Compound nevus
Compound nevus Ajcc 8 melanoma
Ajcc 8 melanoma Abcd of skin cancer
Abcd of skin cancer Nail body
Nail body Melanoma defintion
Melanoma defintion Basal cell carcinoma
Basal cell carcinoma Clark classification of melanoma
Clark classification of melanoma Richard david kann melanoma foundation
Richard david kann melanoma foundation Skin cancer abcde
Skin cancer abcde Nodular melanoma
Nodular melanoma Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Psychoanalytic vs humanistic
Psychoanalytic vs humanistic