ADJUVANT TREATMENT FOR RESECTED MELANOMA DR TOM VAN







- Slides: 11

ADJUVANT TREATMENT FOR RESECTED MELANOMA DR. TOM VAN HAGEN 12 TH NOVEMBER 2019

TAKE HOME MESSAGE For decades, there have been no useful adjuvant treatments that alter the outcome for patients with resected melanoma Until now…


ADJUVANT TREATMENT OPTIONS Observation Interferon Targeted therapy Clinical trial Immunotherapy

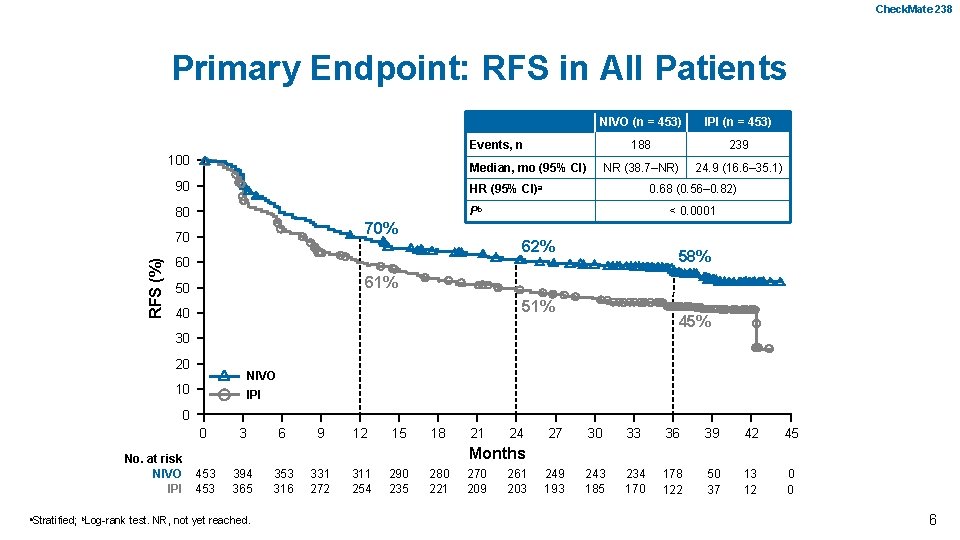
ADJUVANT NIVOLUMAB VERSUS IPILIMUMAB IN RESECTED STAGE III/IV MELANOMA: 3 -YEAR EFFICACY AND BIOMARKER RESULTS FROM THE PHASE 3 CHECKMATE 238 TRIAL JEFFREY WEBER, 1 MICHELE DEL VECCHIO, 2 MARIO MANDALA, 3 HELEN GOGAS, 4 ANA M. ARANCE, 5 STÉPHANE DALLE, 6 C. LANCE COWEY, 7 MICHAEL SCHENKER, 8 JEAN-JACQUES GROB, 9 VANNA CHIARION-SILENI, 10 11 MARCUS BUTLER, 12 13 HAO TANG, 14 ABDEL SACI, 14 IVÁN MÁRQUEZ-RODAS, MICHELE IRCCS MAIO, ISTITUTO 1 NYU PERLMUTTER CANCER CENTER, NEW YORK, NY, USA; 2 FONDAZIONE NAZIONALE DEI TUMORI, MILAN, 3 4 ITALY; PAPA GIOVANNIDE XIIIPRIL, HOSPITAL, BERGAMO, LOBO, ITALY; 14 NATIONAL KAPODISTRIAN UNIVERSITY OF ATHENS, 14 MAURICE 15* PAOLO 16* VEERLE JAMES AND LARKIN, A. ASCIERTO 5 GREECE; HOSPITAL CLÍNIC DE BARCELONA, SPAIN; CIVILS DE LYON, PIERRE BÉNITE, FRANCE; 7 TEXAS ONCOLOGY-BAYLOR CHARLES A. SAMMONS CANCER CENTER, 8 DALLAS, TX, USA; ONCOLOGY CENTER SF NECTARIE LTD. , CRAIOVA, ROMANIA; 9 HÔPITAL DE LA TIMONE, MARSEILLE, FRANCE; 10 VENETO INSTITUTE OF ONCOLOGY IOV – IRCCS, PADUA, ITALY; 11 GENERAL UNIVERSITY HOSPITAL GREGORIO MARAÑÓN & CIBERONC, MADRID, SPAIN; 12 PRINCESS MARGARET CANCER CENTRE, TORONTO, ON, CANADA; 13 CENTER FOR IMMUNO-ONCOLOGY, UNIVERSITY HOSPITAL OF SIENA, ITALY; 14 BRISTOL-MYERS SQUIBB, PRINCETON, NJ, USA; 15 THE ROYAL MARSDEN NHS FOUNDATION TRUST, LONDON, UK; 16 Abstract Number 2801 ISTITUTO NAZIONALE TUMORI IRCCS FONDAZIONE PASCALE, NAPLES, ITALY 6 HOSPICES

Check. Mate 238 Primary Endpoint: RFS in All Patients NIVO (n = 453) IPI (n = 453) 188 239 NR (38. 7‒NR) 24. 9 (16. 6‒ 35. 1) Events, n 100 Median, mo (95% CI) 90 HR (95% CI)a 80 Pb < 0. 0001 70% 70 RFS (%) 0. 68 (0. 56– 0. 82) 62% 60 58% 61% 50 51% 40 45% 30 20 NIVO 10 IPI 0 0 No. at risk NIVO IPI a. Stratified; b. Log-rank 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 249 193 243 185 234 170 178 122 50 37 13 12 0 0 Months 453 394 365 test. NR, not yet reached. 353 316 331 272 311 254 290 235 280 221 270 209 261 203 6

Check. Mate 238 WA’s currently open adjuvant clinical trial… 7

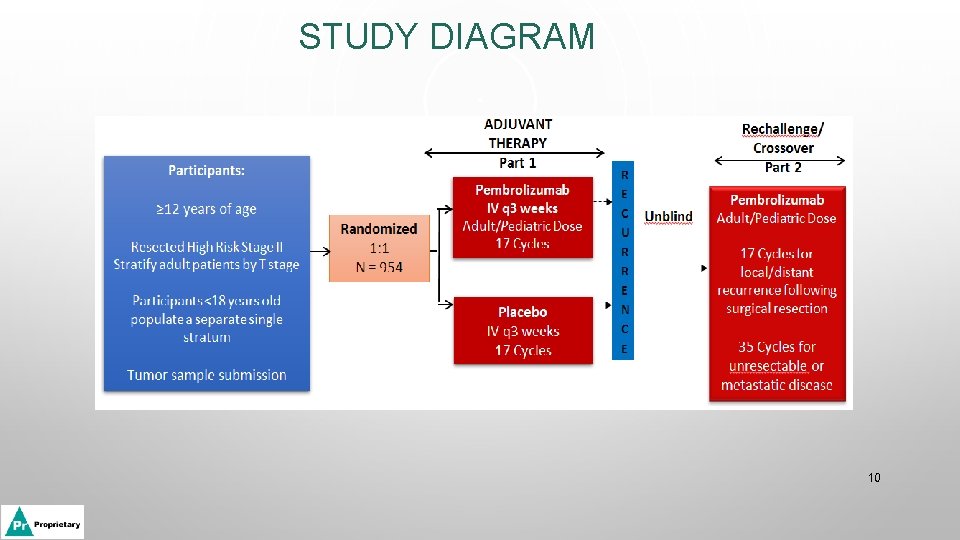
Sponsor Use only: TMF Classification/Site Management/Site Initiation/ Site Training Material MK 3475 -716 Adjuvant Therapy with Pembrolizumab versus Placebo in Resected High risk Stage II Melanoma: A Randomized, Double-blind Phase 3 Study Site Initiation Visit Date: [insert date] 8


STUDY DIAGRAM 10

CONCLUSIO N With many effective adjuvant therapies that improve survival in resected melanoma patients, it is vital that all patients are appropriately staged from the outset