6 1 Notes Stoichiometry Quantitative Chemistry Definitions Stoichiometry





- Slides: 10

6 -1 Notes: Stoichiometry Quantitative Chemistry

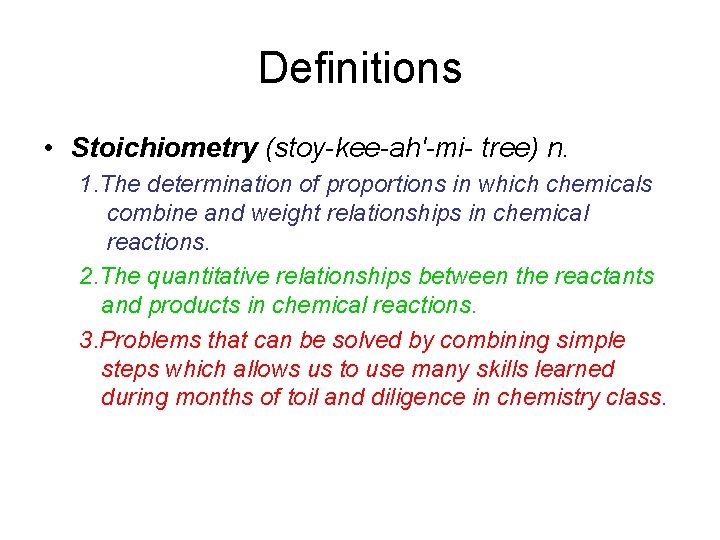
Definitions • Stoichiometry (stoy-kee-ah'-mi- tree) n. 1. The determination of proportions in which chemicals combine and weight relationships in chemical reactions. 2. The quantitative relationships between the reactants and products in chemical reactions. 3. Problems that can be solved by combining simple steps which allows us to use many skills learned during months of toil and diligence in chemistry class.

Hamburger Stoichiometry My recipe for a bacon double cheeseburger: 1 bun (top & bottom) + 2 meat patties + 2 slices of cheese + 4 strips of bacon put them all together… = 1 bacon double cheeseburger

Hamburger Stoichiometry (cont’d) 1 bun (top & bottom) + 2 meat patties + 2 slices of cheese + 4 strips of bacon = 1 bacon double cheeseburger • If I have five bacon double cheeseburgers: – – • How many hamburger buns do I have? How many hamburger patties do I have? How many slices of cheese do I have? How many strips of bacon do I have? How many bacon double cheeseburgers can you make if you start with: – – 1 bun, 2 patties, 2 slices of cheese, 4 strips of bacon 2 buns, 4 patties, 4 slices of cheese, 8 strips of bacon 1 dozen buns, 2 dozen patties, 2 dozen slices of cheese, 4 dozen strips of bacon 1 mole of buns, 2 mole of patties, 2 mole of cheese slices, 4 moles of bacon

Chemical Stoichiometry • My Recipe for Table Salt 2 Sodium Atoms + 1 Chorine Molecule 2 Formula Units of Sodium Chloride – How many formula units can you make if you start with: – 2 Sodium Atoms and 1 Chlorine Molecule – 4 Sodium Atoms and 2 Chlorine Molecules – 2 dozen Sodium Atoms, 1 dozen Chlorine Molecules – 2 moles of Sodium Atoms, 1 mole of Chlorine Molecules

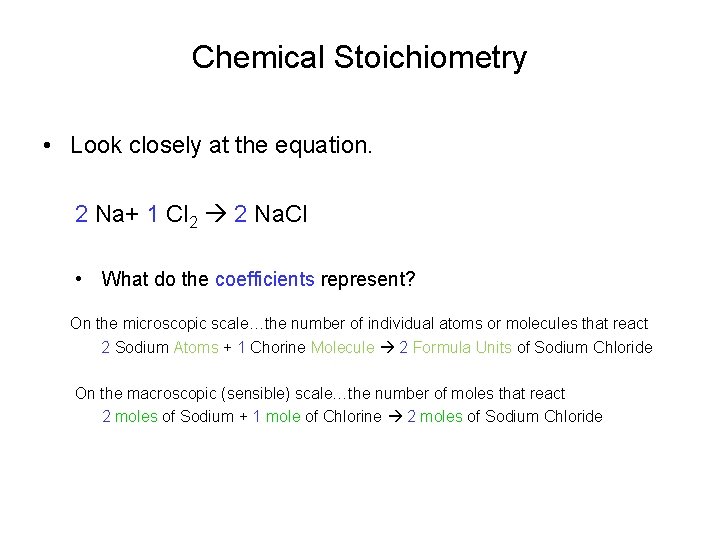
Chemical Stoichiometry • Look closely at the equation. 2 Na+ 1 Cl 2 2 Na. Cl • What do the coefficients represent? On the microscopic scale…the number of individual atoms or molecules that react 2 Sodium Atoms + 1 Chorine Molecule 2 Formula Units of Sodium Chloride On the macroscopic (sensible) scale…the number of moles that react 2 moles of Sodium + 1 mole of Chlorine 2 moles of Sodium Chloride

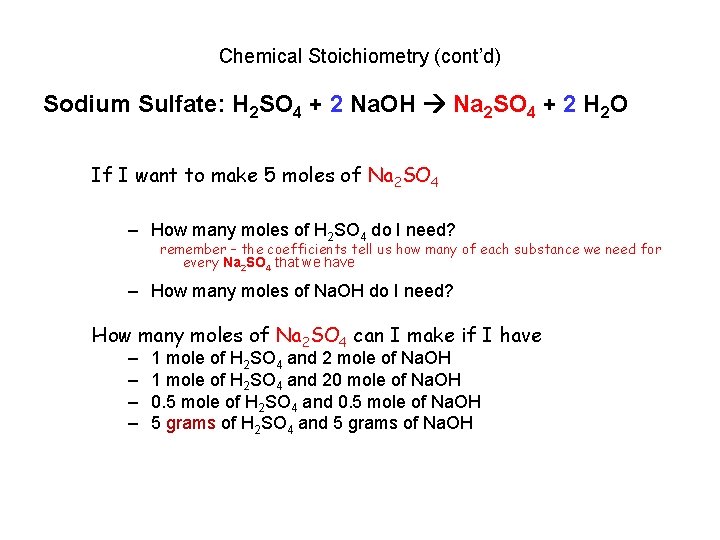
Chemical Stoichiometry (cont’d) Sodium Sulfate: H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O If I want to make 5 moles of Na 2 SO 4 – How many moles of H 2 SO 4 do I need? remember – the coefficients tell us how many of each substance we need for every Na 2 SO 4 that we have – How many moles of Na. OH do I need? How many moles of Na 2 SO 4 can I make if I have – – 1 mole of H 2 SO 4 and 2 mole of Na. OH 1 mole of H 2 SO 4 and 20 mole of Na. OH 0. 5 mole of H 2 SO 4 and 0. 5 mole of Na. OH 5 grams of H 2 SO 4 and 5 grams of Na. OH

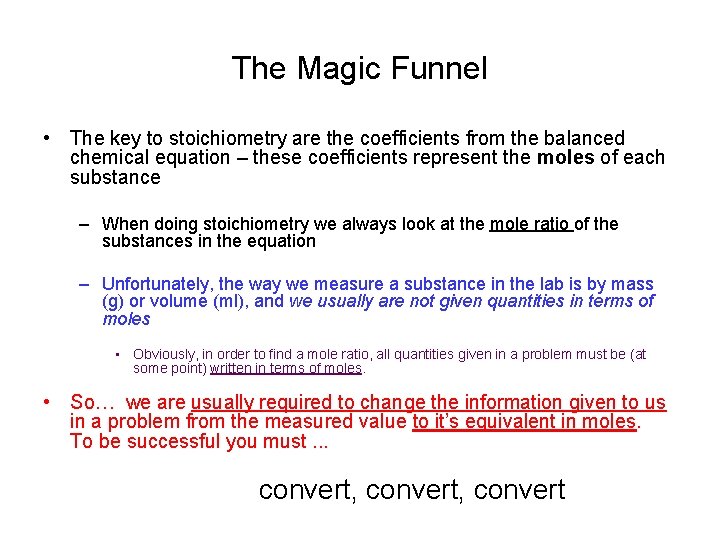
The Magic Funnel • The key to stoichiometry are the coefficients from the balanced chemical equation – these coefficients represent the moles of each substance – When doing stoichiometry we always look at the mole ratio of the substances in the equation – Unfortunately, the way we measure a substance in the lab is by mass (g) or volume (ml), and we usually are not given quantities in terms of moles • Obviously, in order to find a mole ratio, all quantities given in a problem must be (at some point) written in terms of moles. • So… we are usually required to change the information given to us in a problem from the measured value to it’s equivalent in moles. To be successful you must. . . convert, convert

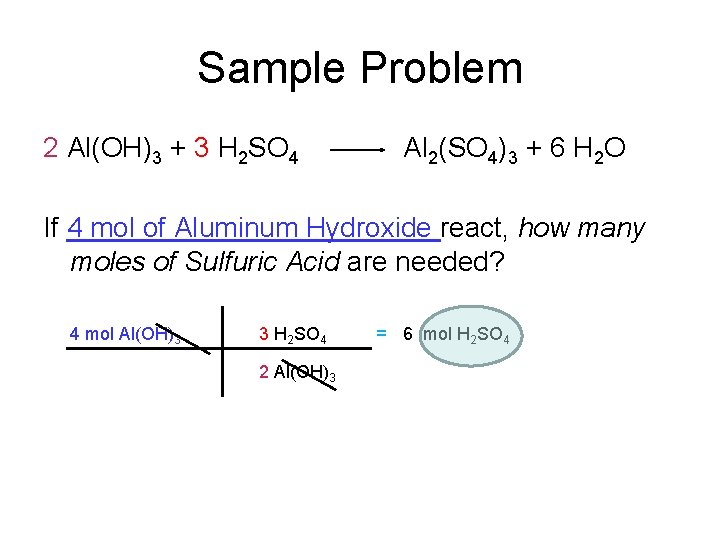
Sample Problem 2 Al(OH)3 + 3 H 2 SO 4 Al 2(SO 4)3 + 6 H 2 O If 4 mol of Aluminum Hydroxide react, how many moles of Sulfuric Acid are needed? 4 mol Al(OH)3 3 H 2 SO 4 2 Al(OH)3 = 6 mol H 2 SO 4

Homework • Chapter 6 Lesson 1 pg 225