STOICHIOMETRY What is stoichiometry Stoichiometry is the quantitative






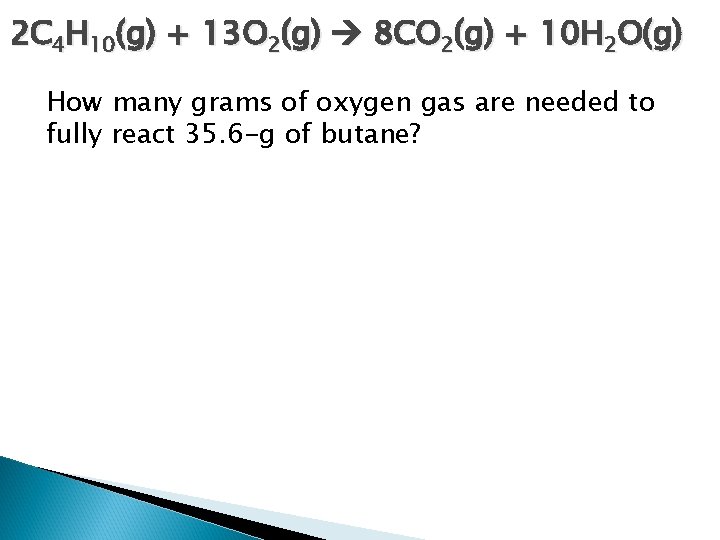
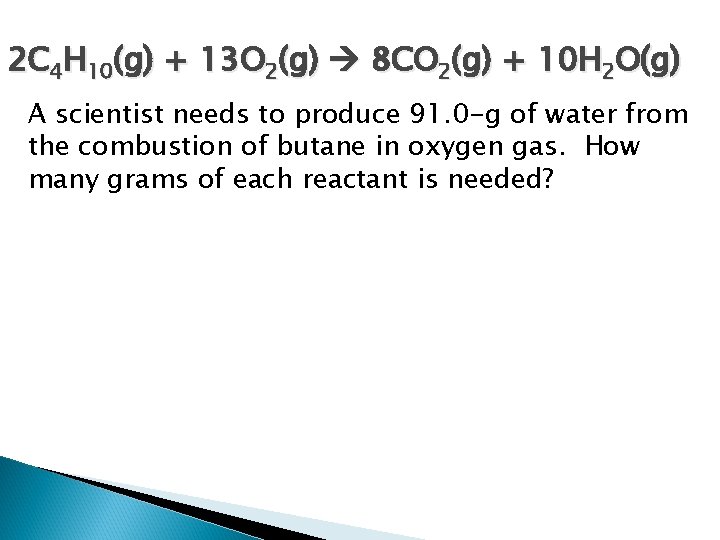


- Slides: 18

STOICHIOMETRY

What is stoichiometry? Stoichiometry is the quantitative study of reactants and products in a chemical reaction.

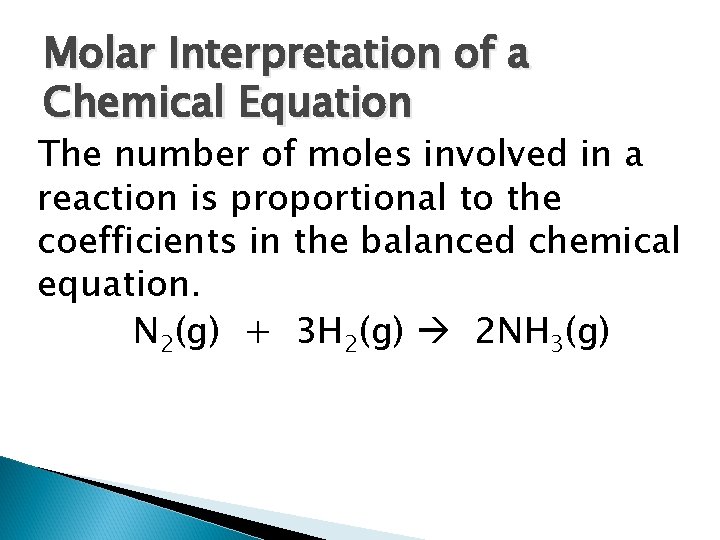
Molar Interpretation of a Chemical Equation The number of moles involved in a reaction is proportional to the coefficients in the balanced chemical equation. N 2(g) + 3 H 2(g) 2 NH 3(g)

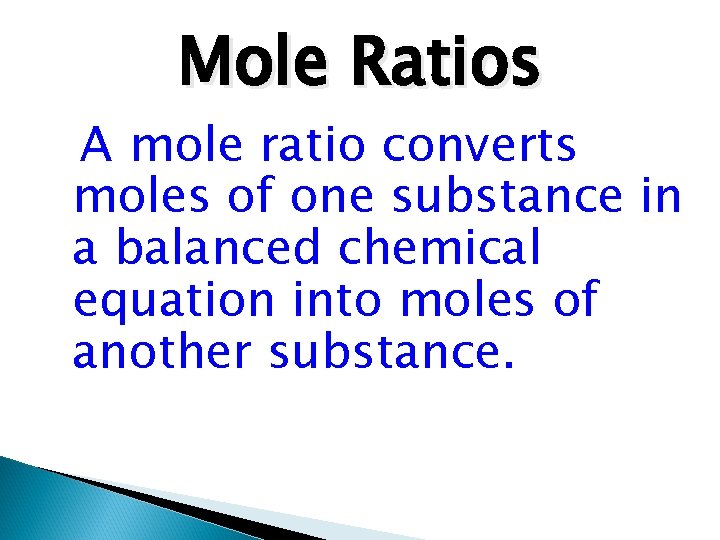
Mole Ratios A mole ratio converts moles of one substance in a balanced chemical equation into moles of another substance.

Mole to Mole Problems The reaction between magnesium and oxygen to form magnesium oxide. Always start with a balanced equation. 2 Mg(s) + O 2(g) 2 Mg. O(s) Calculate the number of moles of magnesium oxide produced by reacting 1. 40 mol of oxygen gas in with excess magnesium.

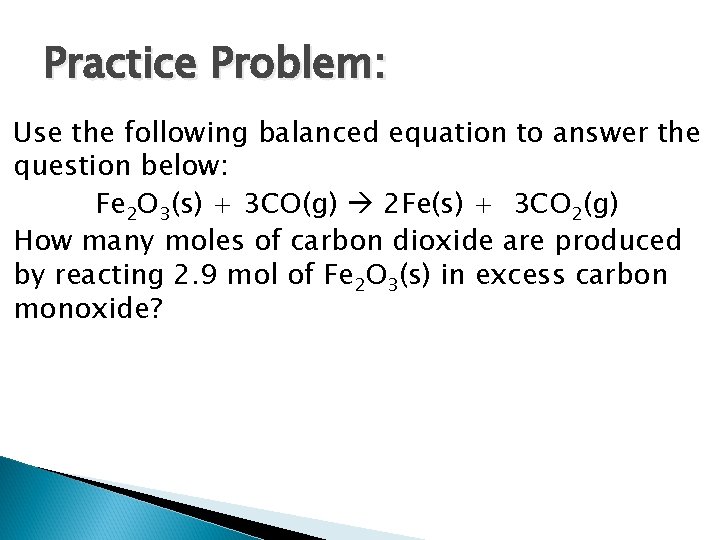
Practice Problem: Use the following balanced equation to answer the question below: Fe 2 O 3(s) + 3 CO(g) 2 Fe(s) + 3 CO 2(g) How many moles of carbon dioxide are produced by reacting 2. 9 mol of Fe 2 O 3(s) in excess carbon monoxide?

Mole – Mass Problems Two Step Stoichiometry

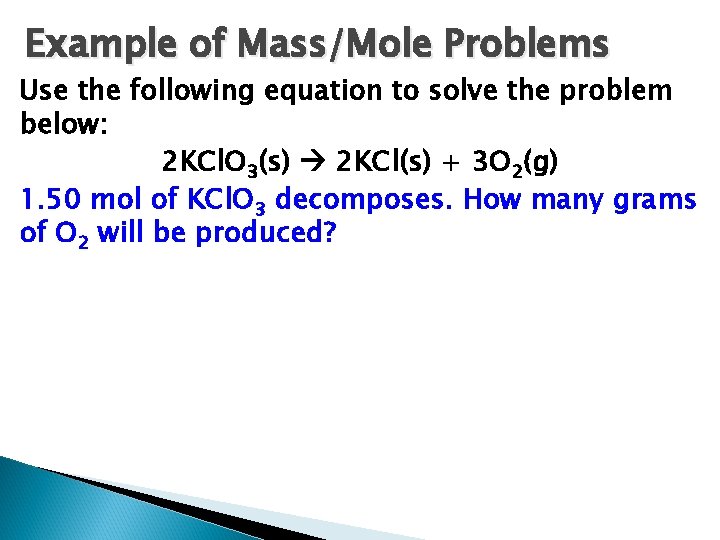
Example of Mass/Mole Problems Use the following equation to solve the problem below: 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g) 1. 50 mol of KCl. O 3 decomposes. How many grams of O 2 will be produced?

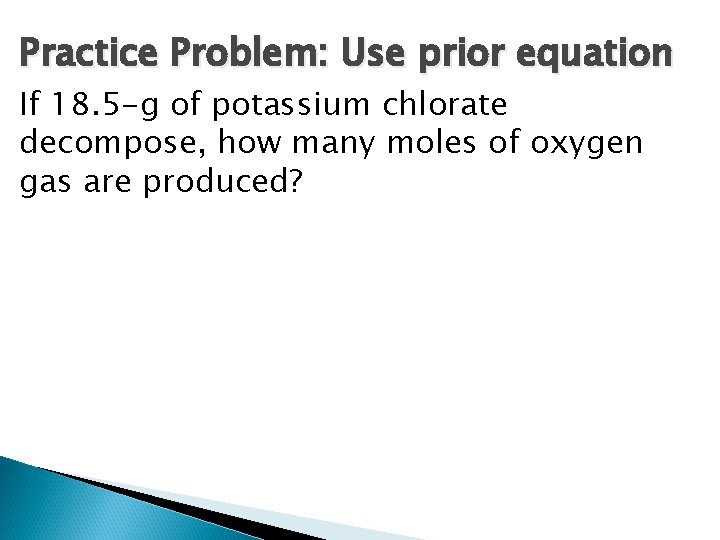
Practice Problem: Use prior equation If 18. 5 -g of potassium chlorate decompose, how many moles of oxygen gas are produced?

Let’s Review: Consider the following equation when solving the problems below: C 6 H 12 O 6(aq) 2 C 2 H 5 OH(ℓ) + 2 CO 2(g) 1. 2. If 2. 5 moles of glucose is decomposed, how many moles of ethyl alcohol is produced? A scientist needs to produce 145. 6 -g of ethyl alcohol. Determine the moles of glucose needed to accomplish this feat.

Mass to mass problems

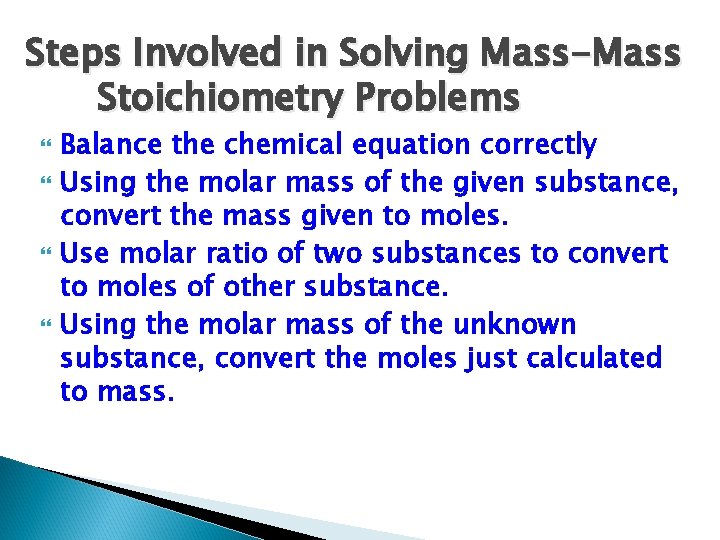
Steps Involved in Solving Mass-Mass Stoichiometry Problems Balance the chemical equation correctly Using the molar mass of the given substance, convert the mass given to moles. Use molar ratio of two substances to convert to moles of other substance. Using the molar mass of the unknown substance, convert the moles just calculated to mass.

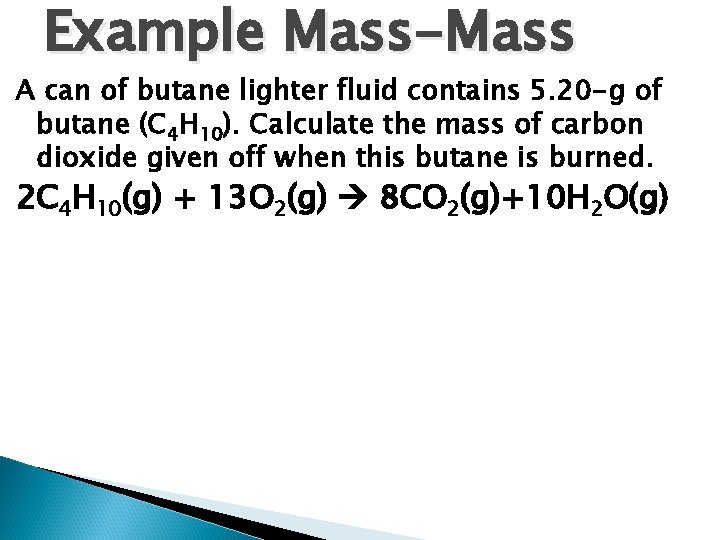
Example Mass-Mass A can of butane lighter fluid contains 5. 20 -g of butane (C 4 H 10). Calculate the mass of carbon dioxide given off when this butane is burned. 2 C 4 H 10(g) + 13 O 2(g) 8 CO 2(g)+10 H 2 O(g)
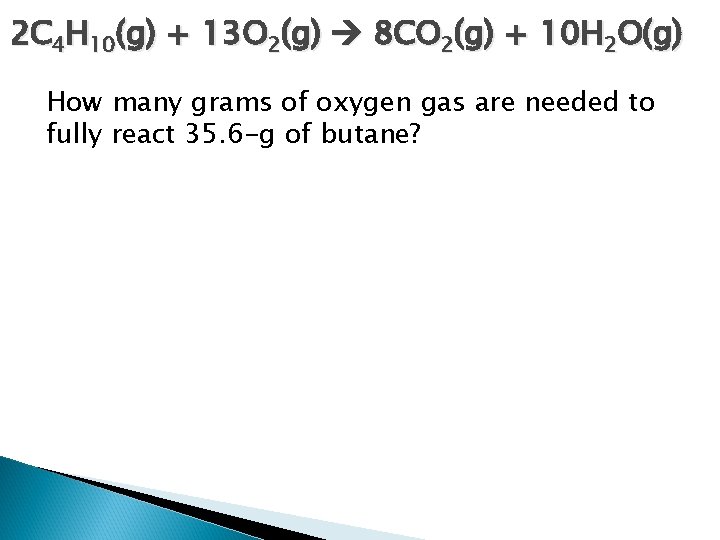
2 C 4 H 10(g) + 13 O 2(g) 8 CO 2(g) + 10 H 2 O(g) How many grams of oxygen gas are needed to fully react 35. 6 -g of butane?
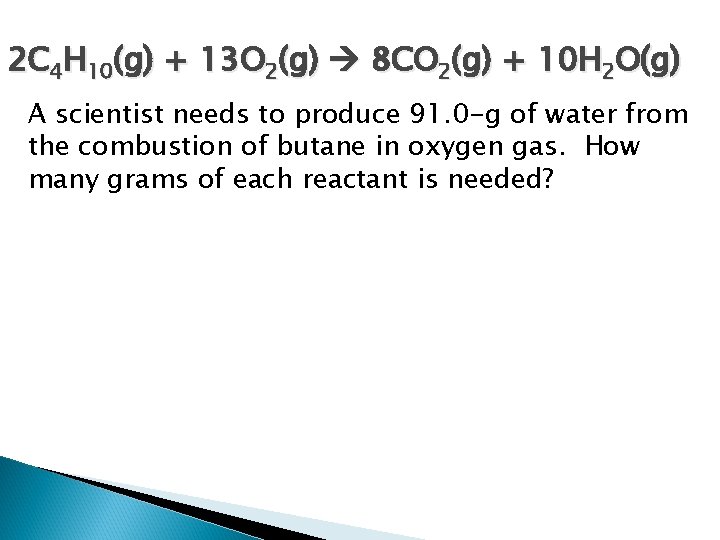
2 C 4 H 10(g) + 13 O 2(g) 8 CO 2(g) + 10 H 2 O(g) A scientist needs to produce 91. 0 -g of water from the combustion of butane in oxygen gas. How many grams of each reactant is needed?

Review: Mass to Mass Nitric acid is formed by the reaction of nitrogen dioxide and water. 3 NO 2(g) + H 2 O(l) NO(g) + 2 HNO 3(aq) If a student reacts 63. 9 -g of NO 2 gas in excess water, how many grams of HNO 3 will be produced?

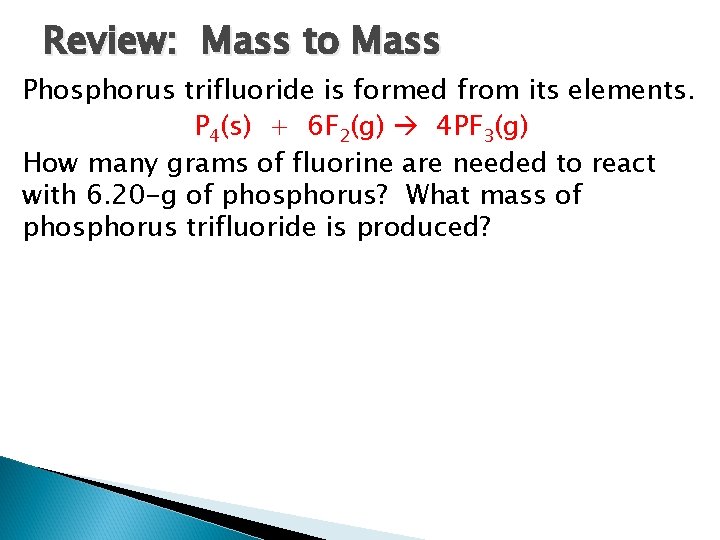
Review: Mass to Mass Phosphorus trifluoride is formed from its elements. P 4(s) + 6 F 2(g) 4 PF 3(g) How many grams of fluorine are needed to react with 6. 20 -g of phosphorus? What mass of phosphorus trifluoride is produced?

Random Stoichiometry questions