2 7 Physical Properties of Alkenes 1 Nonpolar






- Slides: 16

2. 7 Physical Properties of Alkenes 1

• • Nonpolar Insoluble in water Soluble in nonpolar organic solvents. Less dense than water: they float on water. PHYSICAL PROPERTIES LIKE ALKANES 2

2. 8 Chemical Properties of Alkenes 3

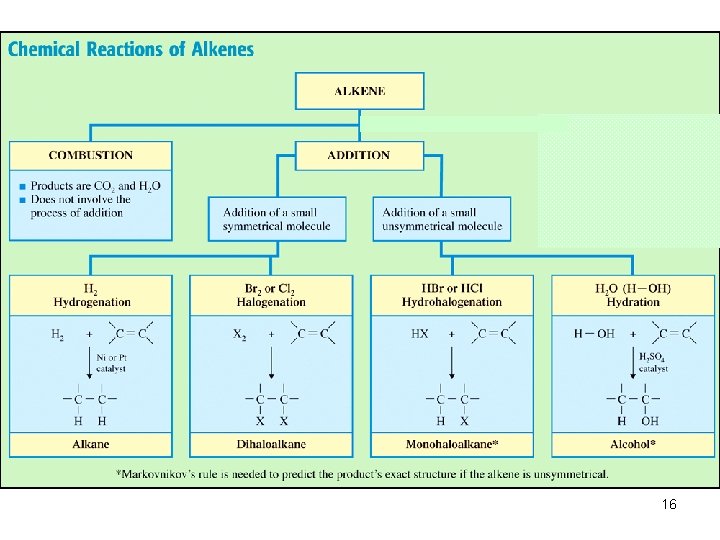
REACTIONS OF ALKENES ØCombustion ØAddition reactions : ühydrogenation ühalogenation ühydrohalogenation ühydration (addition of water) 4

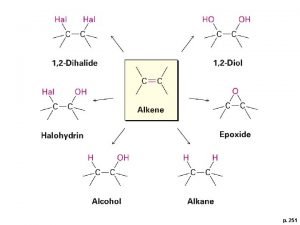
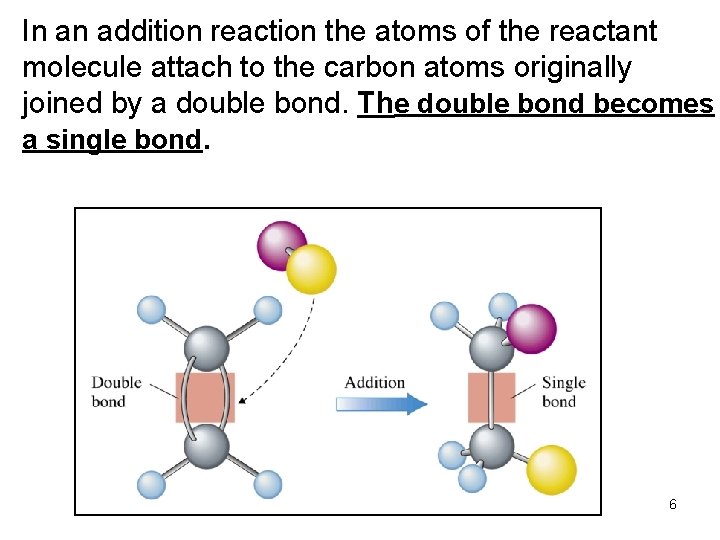
• Addition reactions: A substance adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds. 5

In an addition reaction the atoms of the reactant molecule attach to the carbon atoms originally joined by a double bond. The double bond becomes a single bond. 6

1. Symmetrical Addition Reactions: In which identical atoms are added to each carbon of the double bond. 2. Unsymmetrical Addition Reactions: In which different groups are added to each carbon of the double bond. For example, hydration or hydrohalogenation. 7

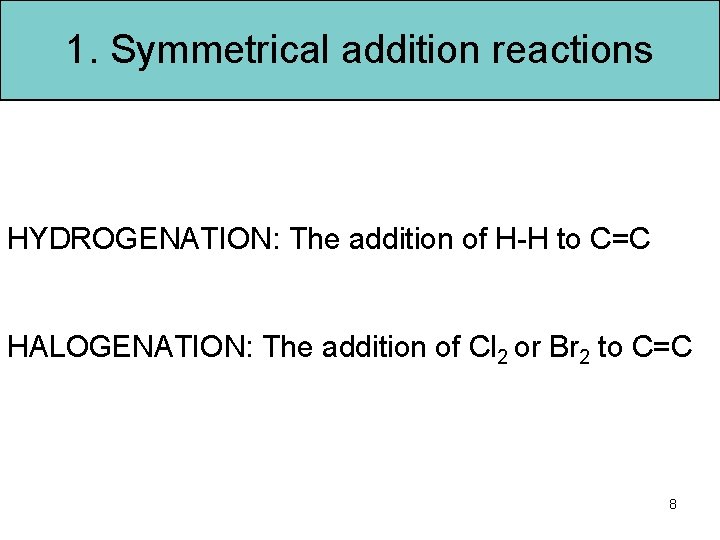
1. Symmetrical addition reactions HYDROGENATION: The addition of H-H to C=C HALOGENATION: The addition of Cl 2 or Br 2 to C=C 8

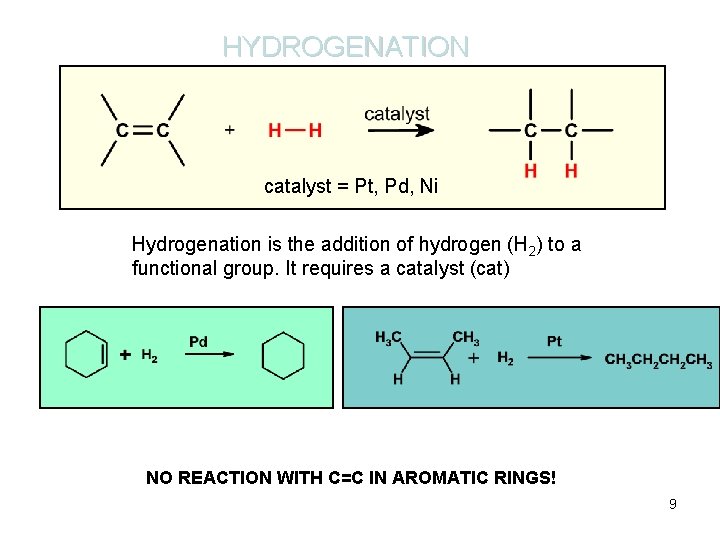
HYDROGENATION catalyst = Pt, Pd, Ni Hydrogenation is the addition of hydrogen (H 2) to a functional group. It requires a catalyst (cat) NO REACTION WITH C=C IN AROMATIC RINGS! 9

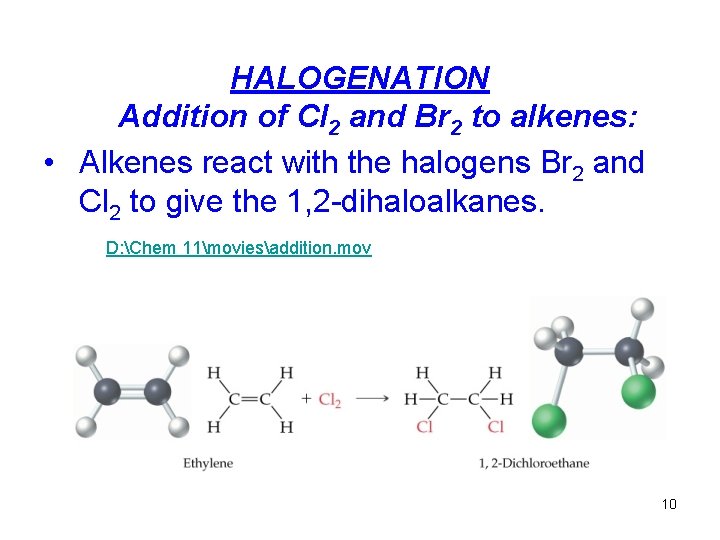
HALOGENATION Addition of Cl 2 and Br 2 to alkenes: • Alkenes react with the halogens Br 2 and Cl 2 to give the 1, 2 -dihaloalkanes. D: Chem 11moviesaddition. mov 10

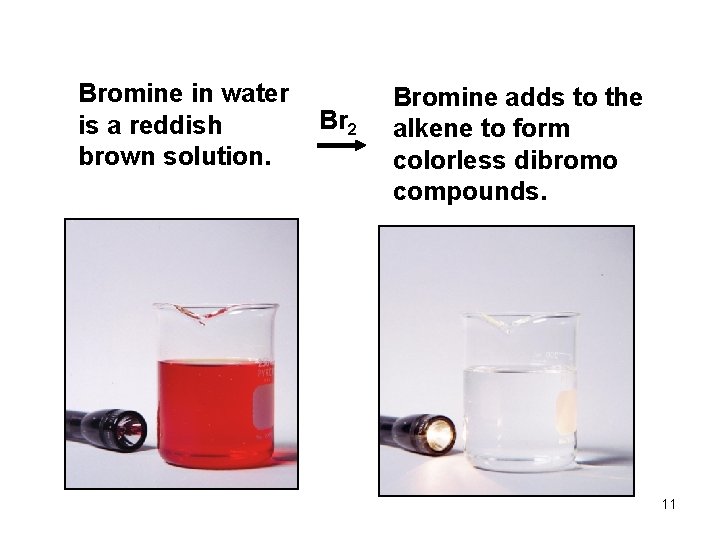
Bromine in water is a reddish brown solution. Br 2 Bromine adds to the alkene to form colorless dibromo compounds. 11

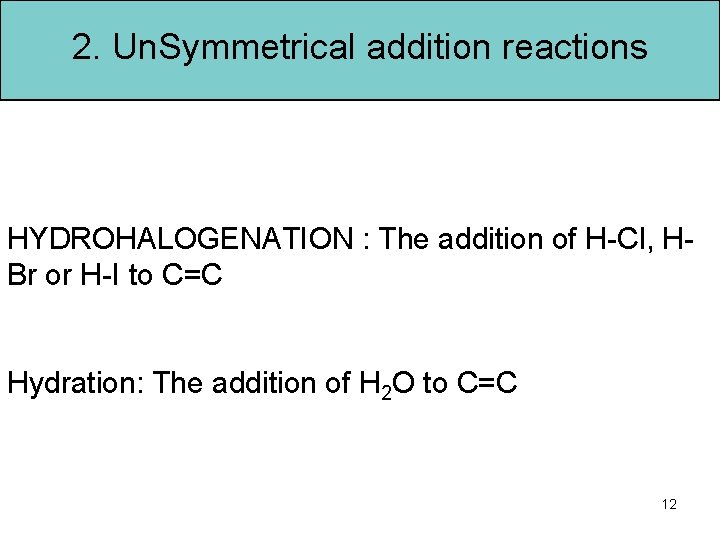
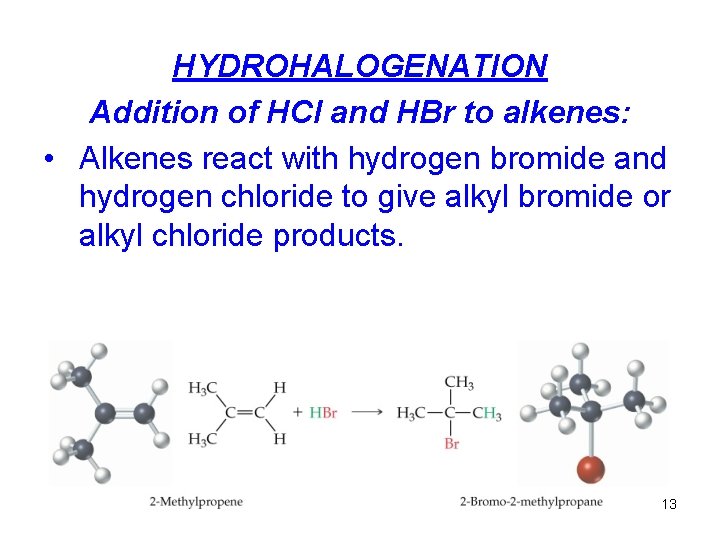
2. Un. Symmetrical addition reactions HYDROHALOGENATION : The addition of H-Cl, HBr or H-I to C=C Hydration: The addition of H 2 O to C=C 12

HYDROHALOGENATION Addition of HCl and HBr to alkenes: • Alkenes react with hydrogen bromide and hydrogen chloride to give alkyl bromide or alkyl chloride products. 13

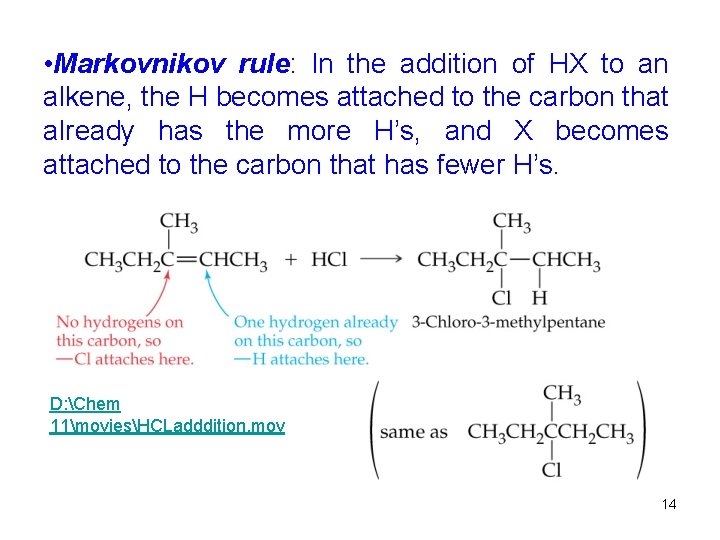
• Markovnikov rule: In the addition of HX to an alkene, the H becomes attached to the carbon that already has the more H’s, and X becomes attached to the carbon that has fewer H’s. D: Chem 11moviesHCLadddition. mov 14

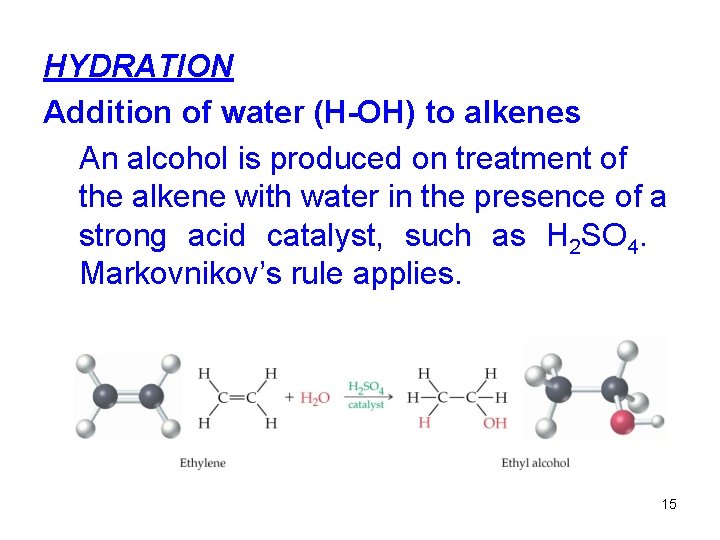
HYDRATION Addition of water (H-OH) to alkenes An alcohol is produced on treatment of the alkene with water in the presence of a strong acid catalyst, such as H 2 SO 4. Markovnikov’s rule applies. 15

16
 Physical properties of alkenes
Physical properties of alkenes Physical properties of alkenes
Physical properties of alkenes Chemical and physical properties
Chemical and physical properties Addition polymerization
Addition polymerization Alkane methane structure
Alkane methane structure 2-iodo-3-methylbutane + aqueous naoh
2-iodo-3-methylbutane + aqueous naoh Syn addition
Syn addition Syn addition
Syn addition Halogenation of alkenes
Halogenation of alkenes What is the general formula for alkenes?
What is the general formula for alkenes? Alkanes alkenes alkynes
Alkanes alkenes alkynes Sp2 hybridization in alkenes
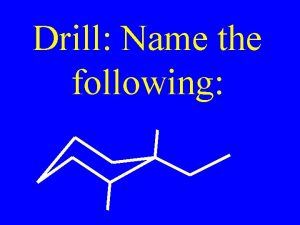
Sp2 hybridization in alkenes Name the following alkenes
Name the following alkenes Alkyne prefix
Alkyne prefix Diol formation from alkene
Diol formation from alkene Bromine water test
Bromine water test Define organic chemistry
Define organic chemistry