10 1 Acids and alkalis in daily life


















- Slides: 33

10. 1 Acids and alkalis in daily life

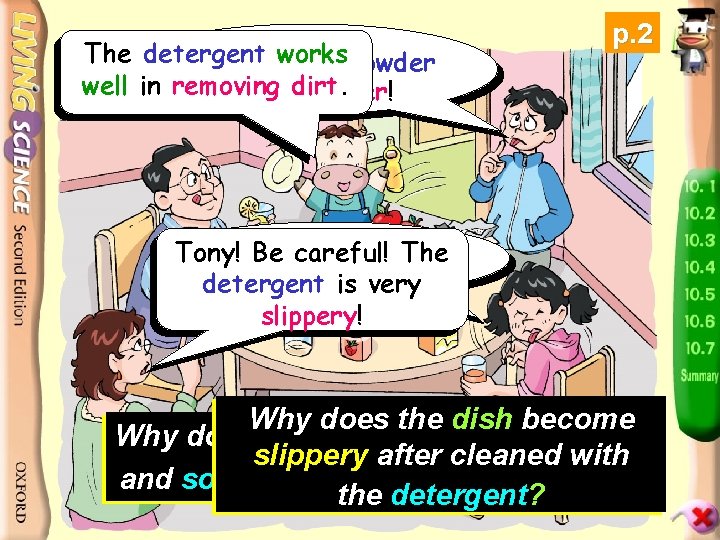
The detergent workspowder The baking well in removing tastesdirt. bitter! p. 2 The Tony! Beorange careful!juice The tastes issour! detergent very slippery! Why does the dish become Why do some substances taste sour slippery after cleaned with and some substances taste bitter? the detergent?

In Unit 6, we have learnt how to classify substances as solids, liquids and gases. In fact, there are many different ways to classify substances. p. 3

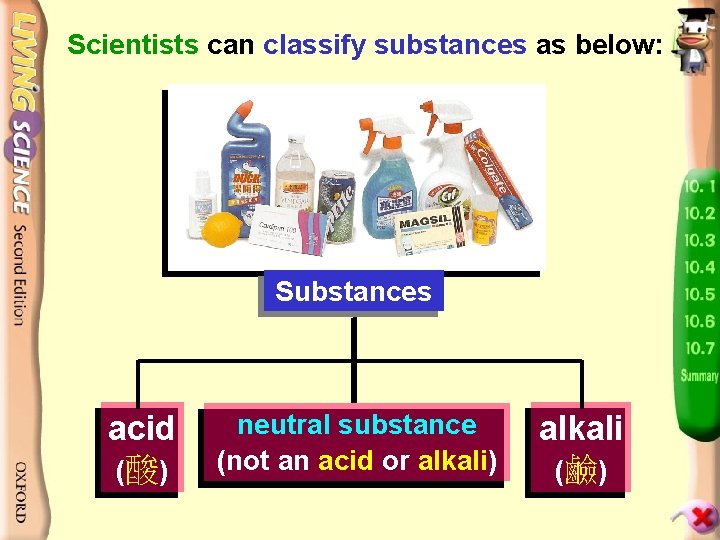
Scientists can classify substances as below: Substances acid (酸) neutral substance (not an acid or alkali) alkali (鹼)

- Many substances in daily life contain acids or alkalis. - Some foodstuffs taste sour because they contain acids. p. 4

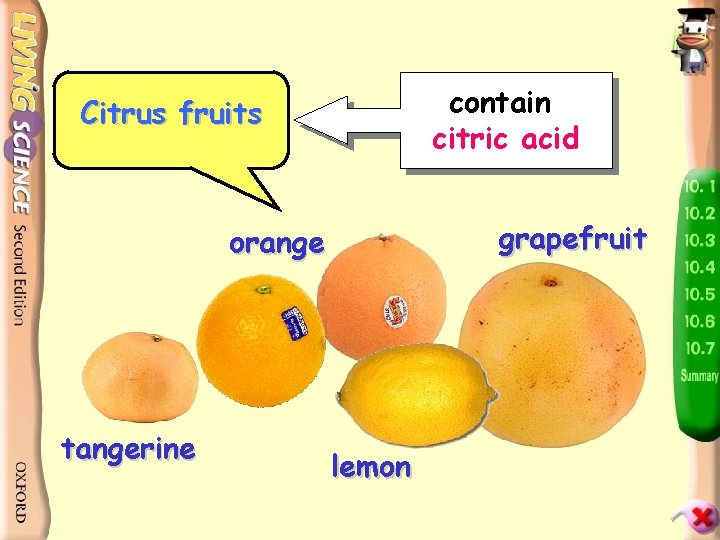
contain citric acid Citrus fruits grapefruit orange tangerine lemon

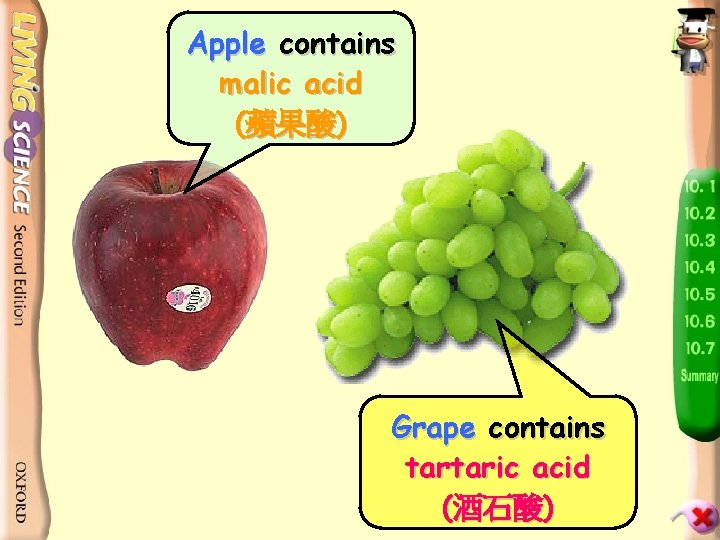
Apple contains malic acid (蘋果酸) Grape contains tartaric acid (酒石酸)

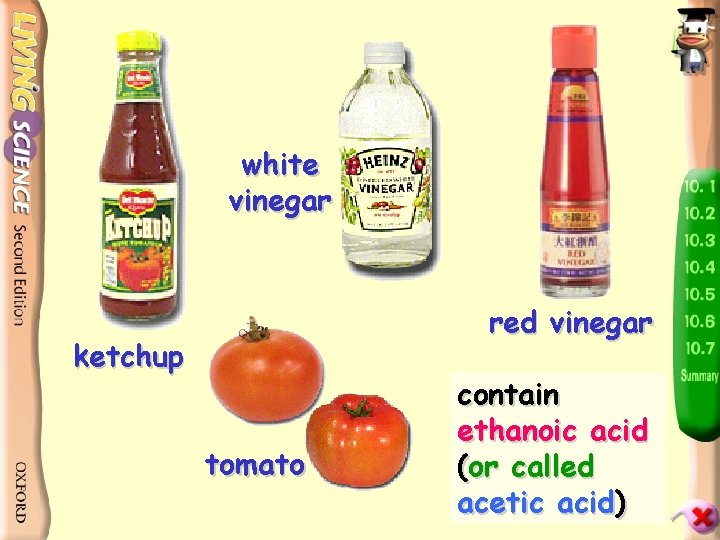
white vinegar red vinegar ketchup tomato contain ethanoic acid (or called acetic acid)

Some soft drinks contain carbonic acid

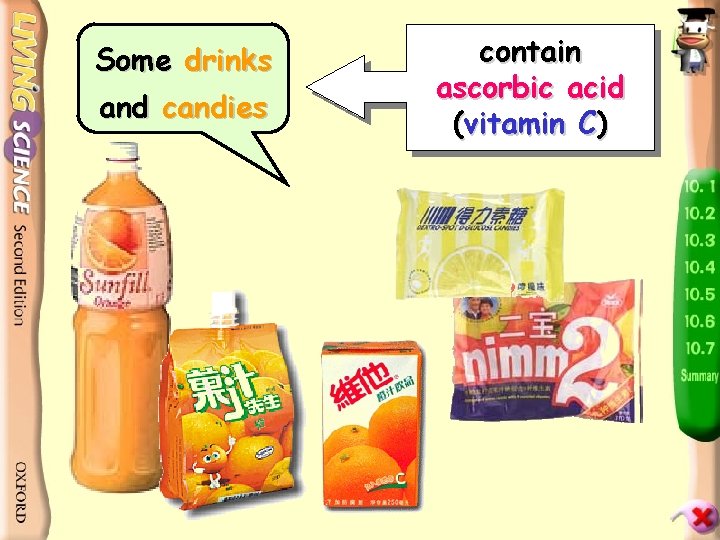
Some drinks and candies contain ascorbic acid (vitamin C)

Milk and yoghurt contain lactic acid

Spinach and some shampoos contain oxalic acid (草酸)

Toilet cleanser contains hydrochloric acid Aspirin also contains acid

p. 4 - Acids taste sour, sour but alkalis generally taste bitter. Did you taste the following alkaline drugs or foodstuff before? How do they taste? antacid almond

- Many household cleansers contain alkalis. Alkalis are good for removing greases. They react with the greases so that the resulting substances can be washed away with water easily.

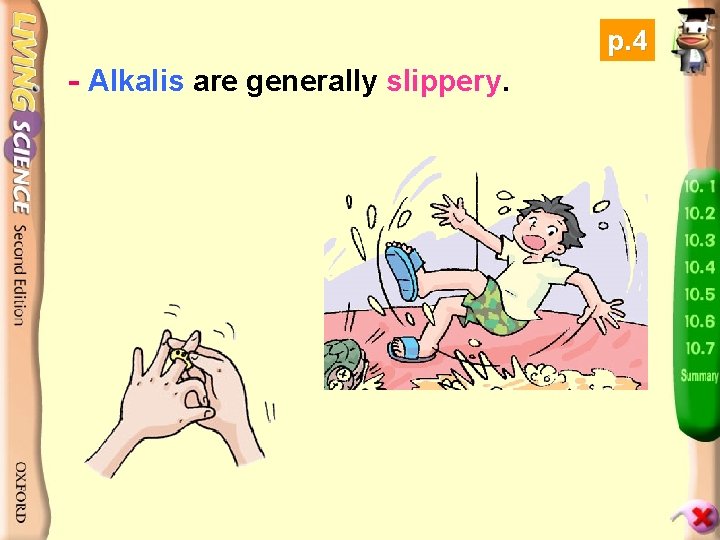
Slippery! Be careful! After the toilet has just been cleaned, there is often a cautionary notice. Why?

p. 4 - Alkalis are generally slippery.

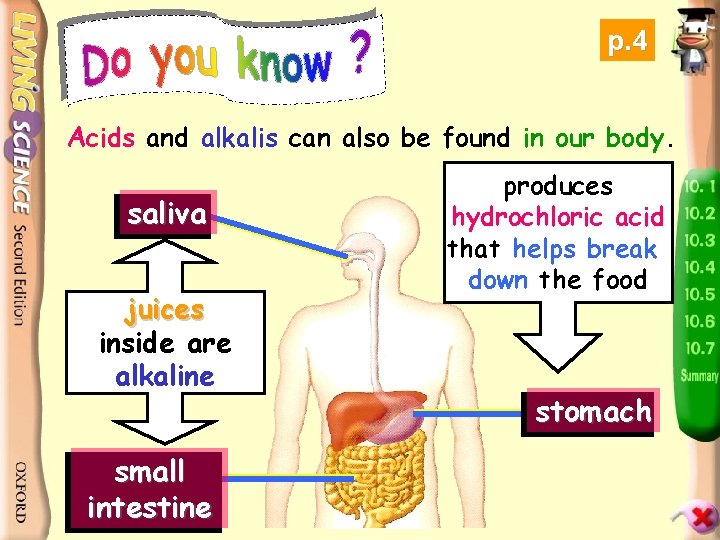
p. 4 Acids and alkalis can also be found in our body. saliva juices inside are alkaline small intestine produces hydrochloric acid that helps break down the food stomach

- Many household cleansers contain alkalis. but this toilet cleanser contains acid instead. => cannot distinguish between acids and alkalis by their uses

Should we distinguish an acid from an alkali by tasting it?

Natural acid-alkali indicator p. 5 - We should distinguish between acids and alkalis by using indicators (指示劑). - Indicators contain pigments => change colour in acids and alkalis. - Some natural materials contain pigments and can be used as natural indicators.

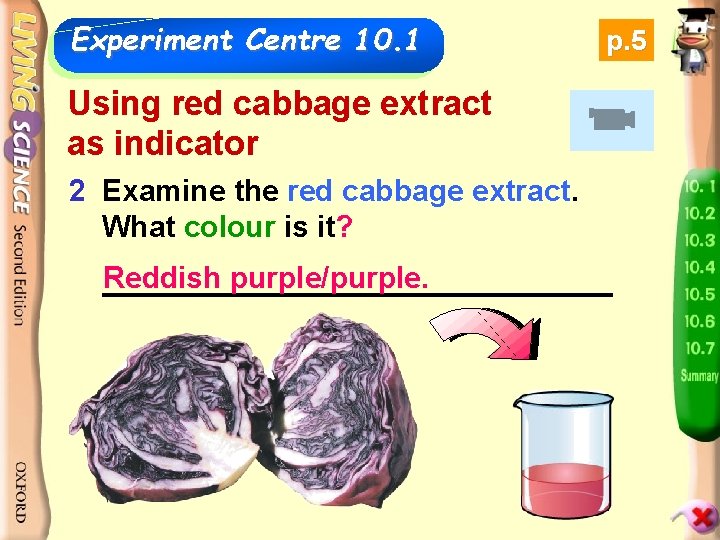
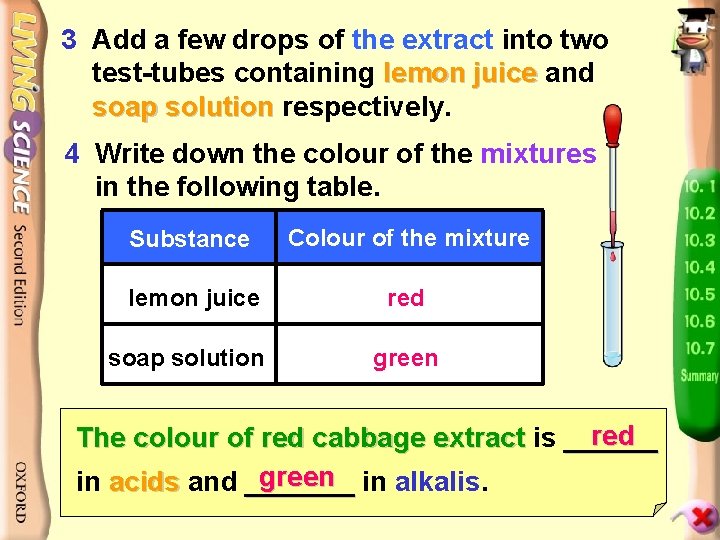
Experiment Centre 10. 1 p. 5 Using red cabbage extract as indicator 2 Cut Examine thecabbage red cabbage extract. 1 the red into pieces and Whatthem colour is it? boil in hot water to get the red cabbage extract. Reddish purple/purple. _______________

3 Add a few drops of the extract into two test-tubes containing lemon juice and soap solution respectively. 4 Write down the colour of the mixtures in the following table. Substance Colour of the mixture lemon juice red soap solution green red The colour of red cabbage extract is ______ green in alkalis. in acids and _______

Little Scientist p. 6 Indicator magic You can also make your own indicator in your kitchen by using the red cabbage. Try to use the indicator to test whether the baking powder solution is acidic or alkaline. baking powder

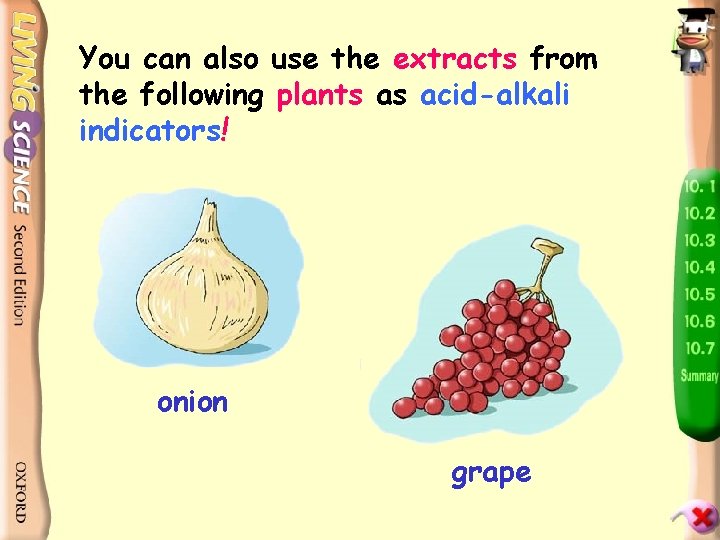
You can also use the extracts from the following plants as acid-alkali indicators! onion grape

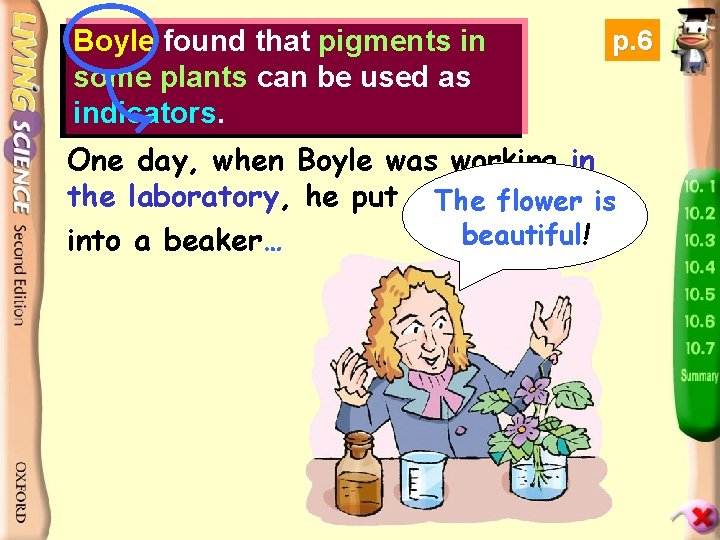
Boyle found that pigments in some plants can be used as indicators. p. 6 One day, when Boyle was (1627– 1691) working in a British scientist the laboratory, he put some The violet flower is beautiful! into a beaker…

When he did some experiments on acids… Oh! The acid splashes on the petals.

Boyle washed the flower with water immediately… The colour of the flower changes from purple to red

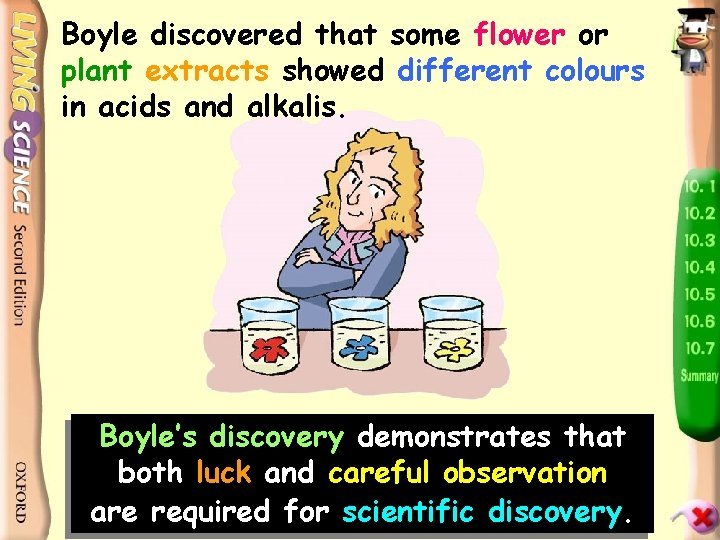
Boyle discovered that some flower or plant extracts showed different colours in acids and alkalis. Boyle’s discovery demonstrates that both luck and careful observation are required for scientific discovery.

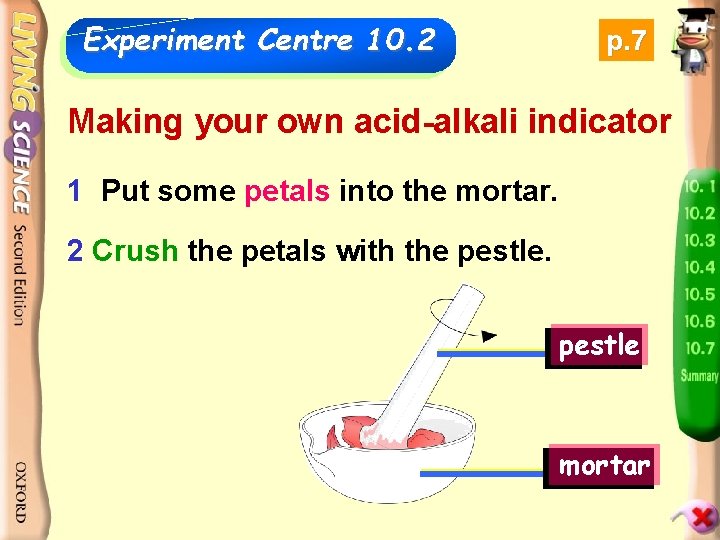
Experiment Centre 10. 2 p. 7 Making your own acid-alkali indicator 1 Put some petals into the mortar. 2 Crush the petals with the pestle mortar

3 Add a few drops of ethanol, mix it with the petals. 4 Add some distilled water so that the volume is enough for three tests. 5 Pour the coloured extract into three test-tubes. petals mixed with ethanol and distilled water

6 Add 2 to 3 drops of lemon juice to one test-tube, soap solution to the second one. The last test-tube contains the extract in distilled water only. 7 Shake the test-tubes and observe the colour of the mixtures. 8 Record your results in the table below. 9 Wash the apparatus. Repeat steps 1 to 8 with petals of other flowers.

Questions p. 8 1 Why do we need to have a test-tube containing the extract in distilled water? For comparison of colour when there _______________ is no acid or alkali. _______________ 2 What extract gives the most clear-cut colour changes? _______________
 Salt preparation methods
Salt preparation methods Everyday acids and alkalis
Everyday acids and alkalis Alkalis
Alkalis Dangerous alkalis
Dangerous alkalis Using polynomials in real life
Using polynomials in real life Opportunities and challenges in informational aspect
Opportunities and challenges in informational aspect Daily life in ancient athens
Daily life in ancient athens Daily life and popular culture in the 1950s
Daily life and popular culture in the 1950s Lcm and hcf worksheet
Lcm and hcf worksheet Anomalous expansion of water
Anomalous expansion of water Applications of raman effect in daily life
Applications of raman effect in daily life Example of like parallel forces and unlike parallel forces
Example of like parallel forces and unlike parallel forces What are two components of projectile motion
What are two components of projectile motion Importance of physics
Importance of physics Mayans daily life
Mayans daily life Introduction to mechatronics
Introduction to mechatronics Applications of boyle's law in real life
Applications of boyle's law in real life Two containers
Two containers Percentage in everyday life
Percentage in everyday life Daily routine watch tv
Daily routine watch tv Byzantine empire vs roman empire venn diagram
Byzantine empire vs roman empire venn diagram Applications of boyle's law in real life
Applications of boyle's law in real life Importance of information technology in daily life
Importance of information technology in daily life Olmec warfare
Olmec warfare Daily life of a roman citizen
Daily life of a roman citizen Where is mission santa barbara located
Where is mission santa barbara located Inca daily life
Inca daily life Critical angle
Critical angle Electrolysis of concentrated sulfuric acid
Electrolysis of concentrated sulfuric acid Apa saja contoh file dalam kehidupan sehari-hari
Apa saja contoh file dalam kehidupan sehari-hari Conic real life examples
Conic real life examples Everyday life in colonial virginia
Everyday life in colonial virginia How did the sui dynasty affect daily life in china
How did the sui dynasty affect daily life in china Daily life in the roman city
Daily life in the roman city