10 6 Everyday uses of acids and alkalis




















- Slides: 29

10. 6 Everyday uses of acids and alkalis

- Micro-organisms (e. g. bacteria and moulds) act on food and make it go bad. e. g. the humidity is high - Some micro-organisms may also Bacteriatoxic growsubstances Moulds grow produce which cause on fruit on bread food poisoning (食物中毒).

Why do microorganisms decay food? Because microorganisms absorb nutrients from food and decompose and decay the food

If we eat food with unnecessary micro-organisms, we may have diarrhoea, dizziness, vomit and high temperature etc. These are symptoms of food poisoning. What is food poisoning?

Acids can be used for food preservation - Acids can be used for food preservation (食物防腐). e. g. - Vinegar is commonly used for food preservation. Solution of ethanoic acid (or called acetic acid)

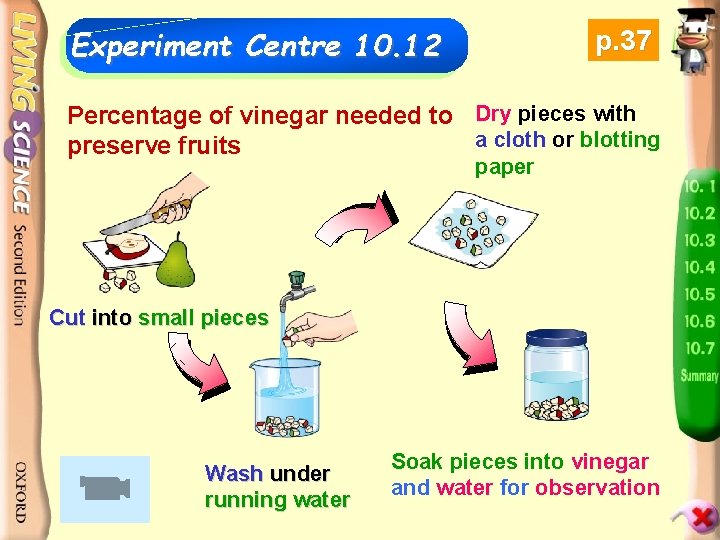
Experiment Centre 10. 12 p. 37 Percentage of vinegar needed to Dry pieces with a cloth or blotting preserve fruits paper Cut into small pieces Wash under running water Soak pieces into vinegar and water for observation

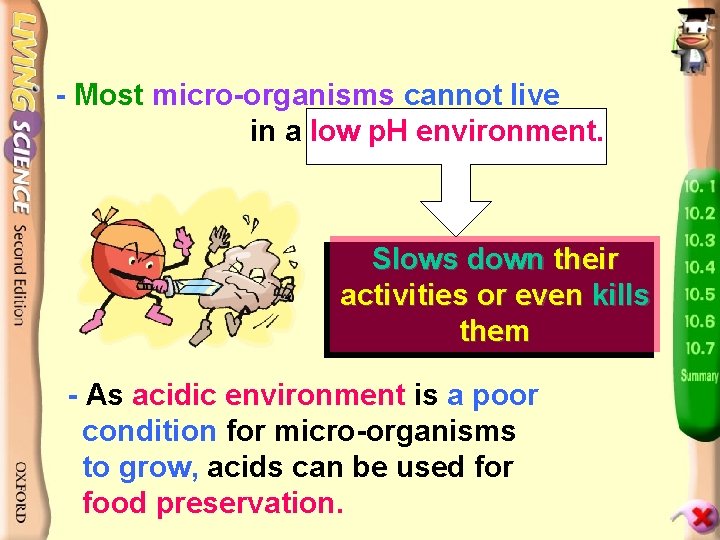
- Most micro-organisms cannot live in a low p. H environment. Slows down their activities or even kills them - As acidic environment is a poor condition for micro-organisms to grow, acids can be used for food preservation.

What are the advantages and disadvantages of using acids for food preservation?

Experiment Centre 10. 13 p. 39 Effect of p. H on browning of fruits Fruits What likesubstances apple, banana, can pear and peach protect turnthe brown fruitwhen better? they are cut and left in the air for a period of time. You can organize your ideas by filling in the investigation planner.

Acids and alkalis as cleansing agents Many cleansing agents contain acids or alkalis. p. 41

Toilet cleanser contains hydrochloric acid Glass cleanser contains ammonia Drain cleanser contains sodium hydroxide

Why can acids and alkalis act as cleansing agents? Acids react with dirts Alkalis react with greases Resulting substances can be wiped or washed away with water easily.

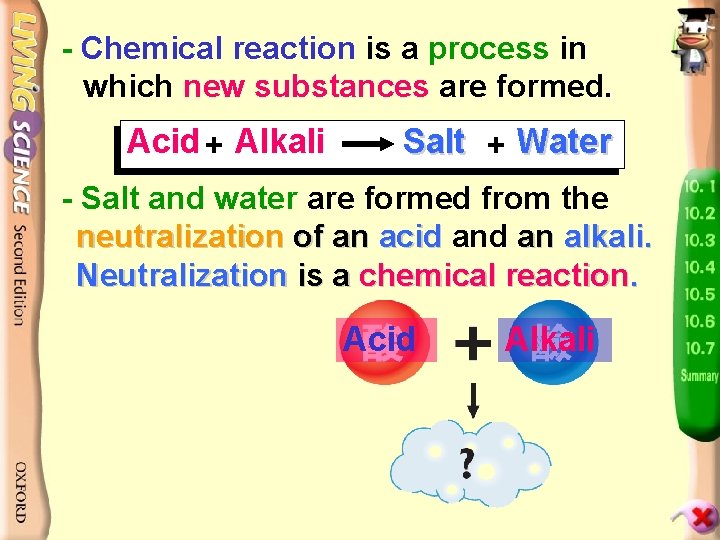
Neutralization(中和作用) p. 42 => Chemical reaction takes place between acids and alkalis Word equation: forming salts and water. Acid + Alkali Salt + Water - In the process of neutralization, the properties of acids and alkalis cancel out each other and the reaction produces neutral salts and water. - If the amounts of acids and alkalis are the same, only salts and water remain after neutralization.

- Chemical reaction is a process in which new substances are formed. Acid + Alkali Salt + Water - Salt and water are formed from the neutralization of an acid an alkali. Neutralization is a chemical reaction. Acid Alkali

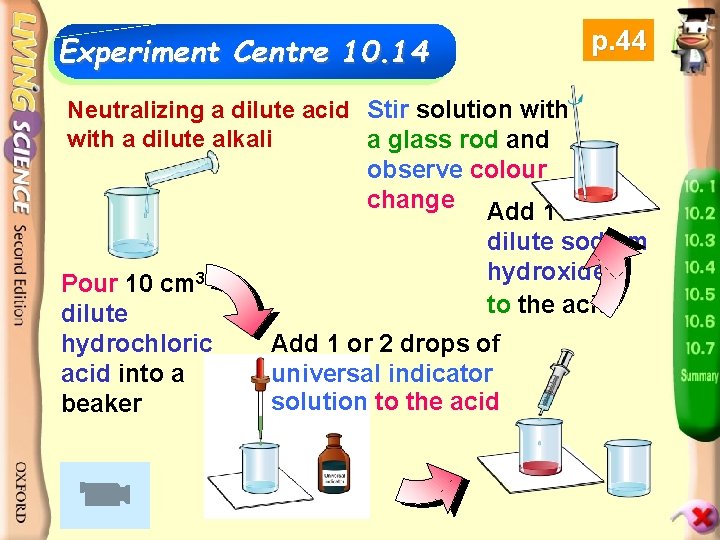
Experiment Centre 10. 14 p. 44 Neutralizing a dilute acid Stir solution with a dilute alkali a glass rod and observe colour change Add 1 cm 3 dilute sodium hydroxide Pour 10 cm 3 to the acid dilute hydrochloric acid into a beaker Add 1 or 2 drops of universal indicator solution to the acid

- Adding acid into alkali or adding alkali into acid can cause neutralization. The products formed are salt and water. Acid + Alkali Salt + Water Why can’t we see the salt in the above experiment? -- When acid is justis neutralized anacid, alkali, When an excess alkali added intoby the neutral is formed. The p. H the p. H solution value increases to above 7. value should be 7. Acid + Alkali Neutral solution

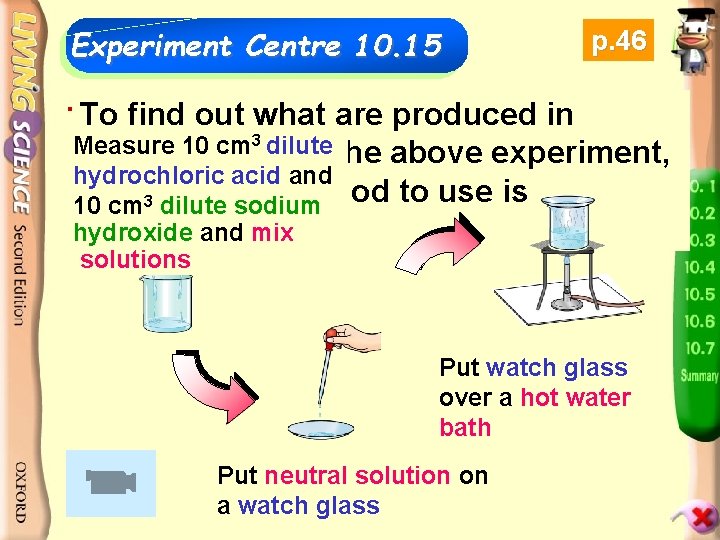
Experiment Centre 10. 15 p. 46 To products find outofwhat are produced in The neutralization 3 dilute Measure 10 cm neutralization in the above experiment, hydrochloric acid and the simplest method to use is 10 cm 3 dilute sodium evaporation. hydroxide and mix solutions Put watch glass over a hot water bath Put neutral solution on a watch glass

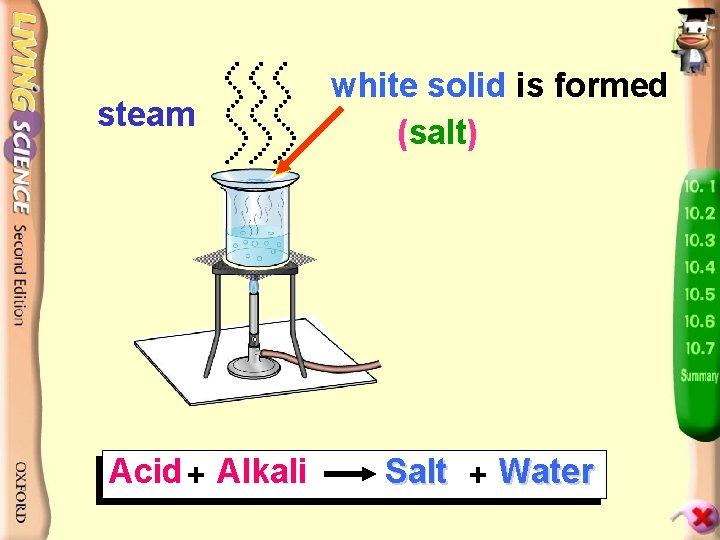
steam Acid + Alkali white solid is formed (salt) Salt + Water

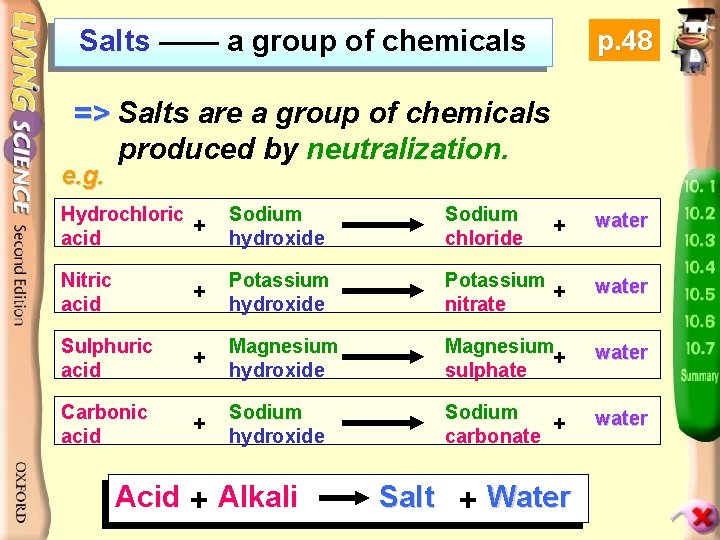
Salts —— a group of chemicals p. 48 => Salts are a group of chemicals produced by neutralization. e. g. Hydrochloric + acid Sodium hydroxide Sodium chloride + water Nitric acid + Potassium hydroxide Potassium + nitrate water Sulphuric acid + Magnesium hydroxide Magnesium + sulphate water Carbonic acid + Sodium hydroxide Sodium + carbonate water Acid + Alkali Salt + Water

- We commonly use salts in our daily life. e. g. Sodium chloride > as table salt > used for production of chlorine and sodium hydroxide

Potassium nitrate > used in matches Magnesium sulphate > used in bath salt

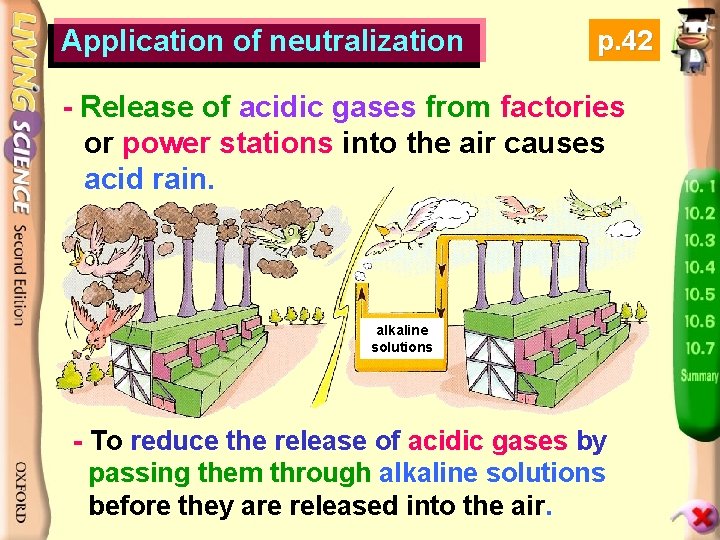
Application of neutralization p. 42 - Release of acidic gases from factories or power stations into the air causes acid rain. alkaline solutions - To reduce the release of acidic gases by passing them through alkaline solutions before they are released into the air.

- Other than helping reduce harmful substances, neutralization can also control the p. H values of substances. e. g. Antacid (制酸劑) > is an alkali. > can neutralize excess acid in our stomach.

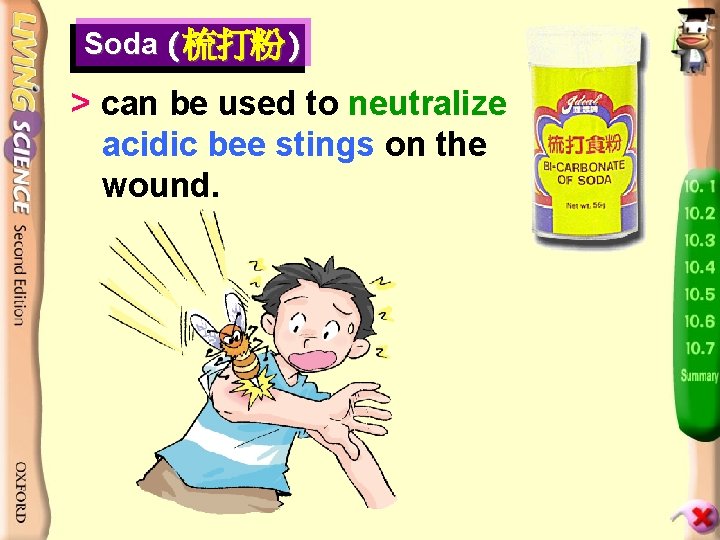
Soda (梳打粉) > can be used to neutralize acidic bee stings on the wound.

Vinegar(醋) > can be used to neutralize alkaline wasp stings on the wound.

Lime(石灰) > is added to acidic soil to raise the p. H value as plants only grow well at particular p. H values.

Mosquito stings leave acidic secretion on the wound. What kind of substances can we use to relieve the pain ?

p. 43 Have you ever eaten glutinous rice dumpling preserved in lye? What is the taste of it? Lye is added in making the dumpling Lye is a natural alkali. It helps neutralize the acidic glutinous rice.

Do you add vinegar when eating a bowl of noodles? Why do we add vinegar to it ? Adding vinegar to a bowl of noodles can reduce the bitter taste by neutralizing the lye. Lye is added to noodles to make them ‘elastic’ to eat. It explains why the noodles taste bitter.
 Everyday alkalis
Everyday alkalis Hso4na
Hso4na Alkali examples
Alkali examples Alkalis feel
Alkalis feel Common acids
Common acids Naming and writing formulas for acids and bases
Naming and writing formulas for acids and bases 5 properties of bases
5 properties of bases Naming and writing formulas for acids and bases
Naming and writing formulas for acids and bases Everyday signs and symbols
Everyday signs and symbols Chapter 6 section 1 price supply and demand together
Chapter 6 section 1 price supply and demand together Health skills definition
Health skills definition Introduction to acids and bases webquest
Introduction to acids and bases webquest Bases examples
Bases examples Weak base strong acid titration curve
Weak base strong acid titration curve Strong acids and bases
Strong acids and bases Always add the word to the end when naming acids
Always add the word to the end when naming acids Arrhenius acid examples
Arrhenius acid examples Modern chemistry chapter 14 review answers
Modern chemistry chapter 14 review answers Characteristics of acids and bases
Characteristics of acids and bases Difference between saturated and unsaturated fatty acids
Difference between saturated and unsaturated fatty acids Purely glucogenic amino acids
Purely glucogenic amino acids How to remember strong acids and strong bases
How to remember strong acids and strong bases Naturally occuring fatty acids
Naturally occuring fatty acids Chapter 19 acids bases and salts answer key
Chapter 19 acids bases and salts answer key Chapter 19 acids bases and salts
Chapter 19 acids bases and salts Chapter 19 acids bases and salts
Chapter 19 acids bases and salts Carboxylic acids and their derivatives
Carboxylic acids and their derivatives Characteristics of acids and bases
Characteristics of acids and bases Characteristics of base
Characteristics of base Chapter 8 solutions acids and bases
Chapter 8 solutions acids and bases