12 Acids and alkalis Lesson starter Indicators Different
- Slides: 2

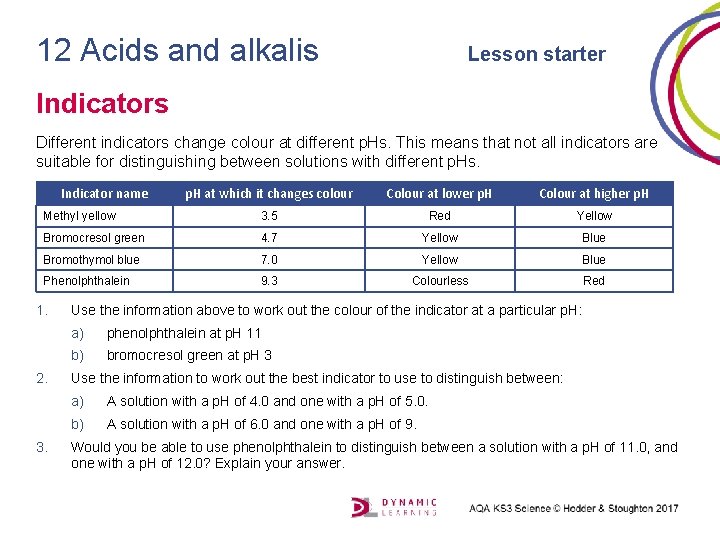
12 Acids and alkalis Lesson starter Indicators Different indicators change colour at different p. Hs. This means that not all indicators are suitable for distinguishing between solutions with different p. Hs. Indicator name p. H at which it changes colour Colour at lower p. H Colour at higher p. H Methyl yellow 3. 5 Red Yellow Bromocresol green 4. 7 Yellow Blue Bromothymol blue 7. 0 Yellow Blue Phenolphthalein 9. 3 Colourless Red 1. 2. 3. Use the information above to work out the colour of the indicator at a particular p. H: a) phenolphthalein at p. H 11 b) bromocresol green at p. H 3 Use the information to work out the best indicator to use to distinguish between: a) A solution with a p. H of 4. 0 and one with a p. H of 5. 0. b) A solution with a p. H of 6. 0 and one with a p. H of 9. Would you be able to use phenolphthalein to distinguish between a solution with a p. H of 11. 0, and one with a p. H of 12. 0? Explain your answer.

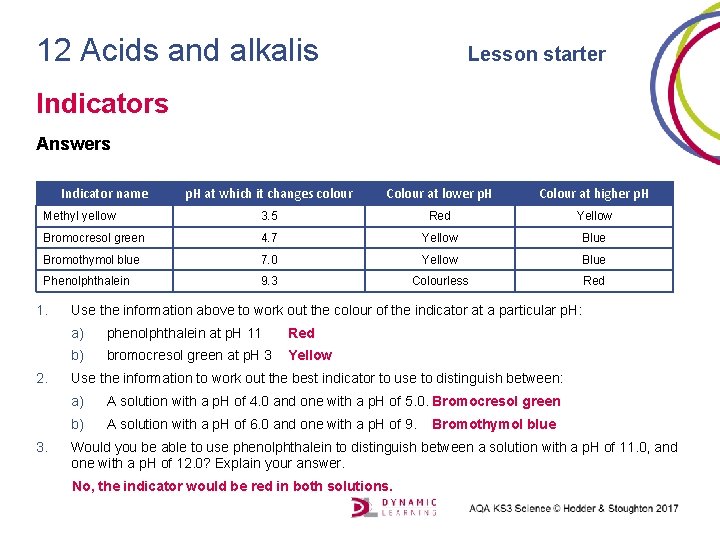
12 Acids and alkalis Lesson starter Indicators Answers Indicator name p. H at which it changes colour Colour at lower p. H Colour at higher p. H Methyl yellow 3. 5 Red Yellow Bromocresol green 4. 7 Yellow Blue Bromothymol blue 7. 0 Yellow Blue Phenolphthalein 9. 3 Colourless Red 1. 2. 3. Use the information above to work out the colour of the indicator at a particular p. H: a) phenolphthalein at p. H 11 Red b) bromocresol green at p. H 3 Yellow Use the information to work out the best indicator to use to distinguish between: a) A solution with a p. H of 4. 0 and one with a p. H of 5. 0. Bromocresol green b) A solution with a p. H of 6. 0 and one with a p. H of 9. Bromothymol blue Would you be able to use phenolphthalein to distinguish between a solution with a p. H of 11. 0, and one with a p. H of 12. 0? Explain your answer. No, the indicator would be red in both solutions.