What are molecules and compounds Molecules are groups


- Slides: 12

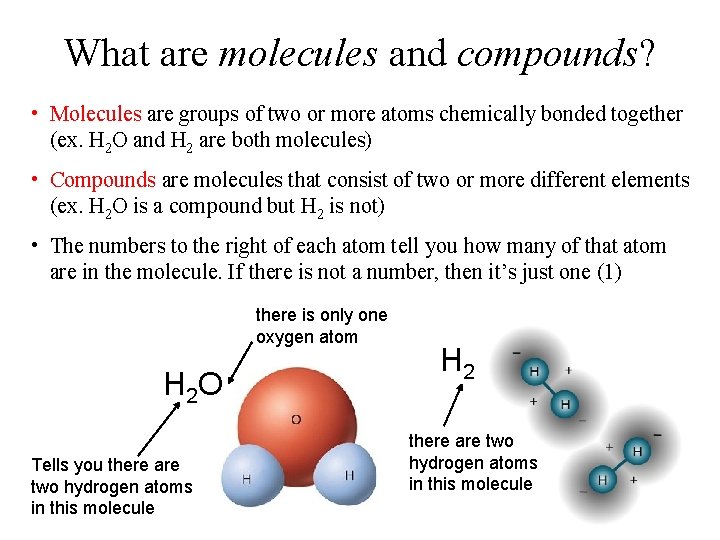
What are molecules and compounds? • Molecules are groups of two or more atoms chemically bonded together (ex. H 2 O and H 2 are both molecules) • Compounds are molecules that consist of two or more different elements (ex. H 2 O is a compound but H 2 is not) • The numbers to the right of each atom tell you how many of that atom are in the molecule. If there is not a number, then it’s just one (1) there is only one oxygen atom H 2 O Tells you there are two hydrogen atoms in this molecule H 2 there are two hydrogen atoms in this molecule

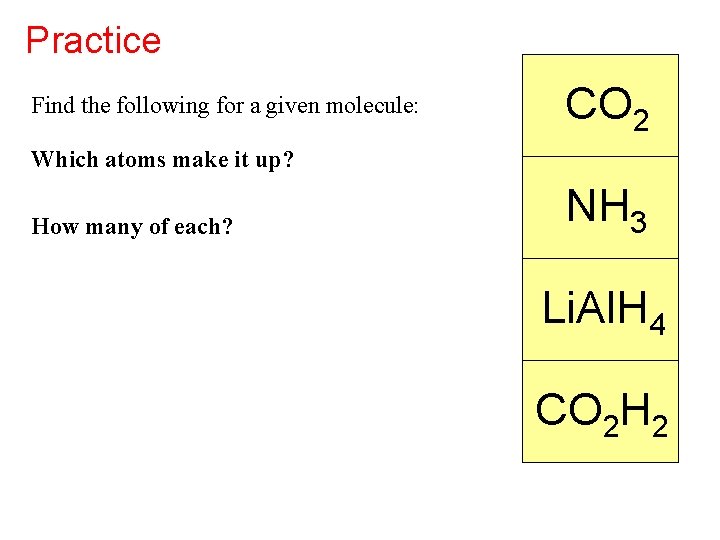
Practice Find the following for a given molecule: CO 2 Which atoms make it up? How many of each? NH 3 Li. Al. H 4 CO 2 H 2

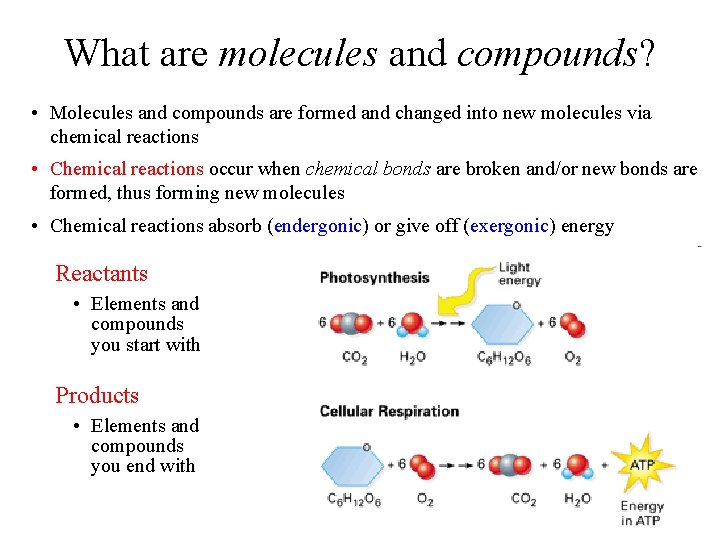
What are molecules and compounds? • Molecules and compounds are formed and changed into new molecules via chemical reactions • Chemical reactions occur when chemical bonds are broken and/or new bonds are formed, thus forming new molecules • Chemical reactions absorb (endergonic) or give off (exergonic) energy Reactants • Elements and compounds you start with Products • Elements and compounds you end with

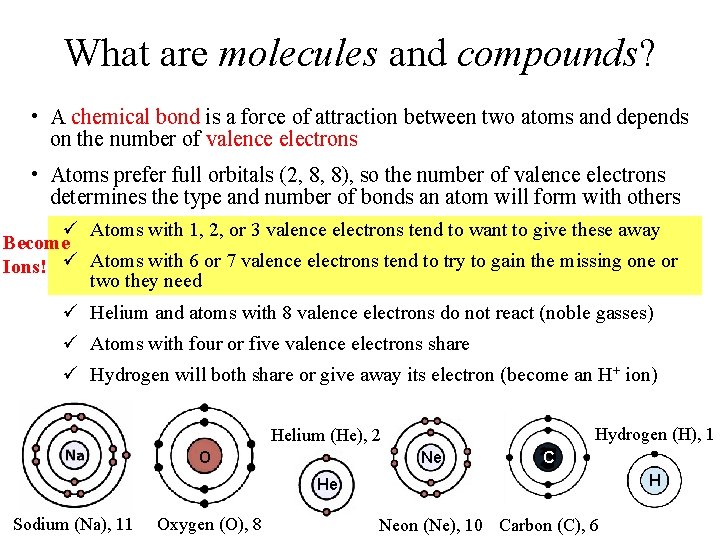
What are molecules and compounds? • A chemical bond is a force of attraction between two atoms and depends on the number of valence electrons • Atoms prefer full orbitals (2, 8, 8), so the number of valence electrons determines the type and number of bonds an atom will form with others ü Atoms with 1, 2, or 3 valence electrons tend to want to give these away Become Ions! ü Atoms with 6 or 7 valence electrons tend to try to gain the missing one or two they need ü Helium and atoms with 8 valence electrons do not react (noble gasses) ü Atoms with four or five valence electrons share ü Hydrogen will both share or give away its electron (become an H+ ion) Hydrogen (H), 1 Helium (He), 2 O Ne C H He Sodium (Na), 11 Oxygen (O), 8 Neon (Ne), 10 Carbon (C), 6

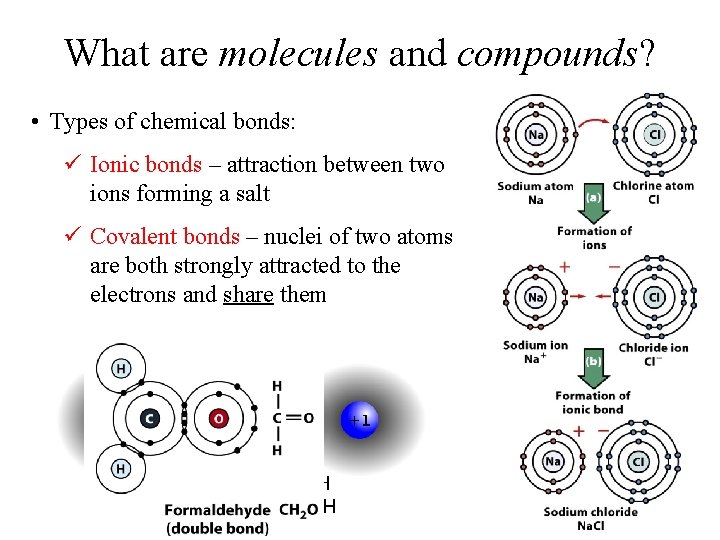
What are molecules and compounds? • Types of chemical bonds: ü Ionic bonds – attraction between two ions forming a salt ü Covalent bonds – nuclei of two atoms are both strongly attracted to the electrons and share them

Bonding examples: http: //www. visionlearning. com/library/module_viewer. php? mid=55 http: //www. mhhe. com/physsci/chemistry/animations/chang_7 e_esp/bom 1 s 2_11. swf

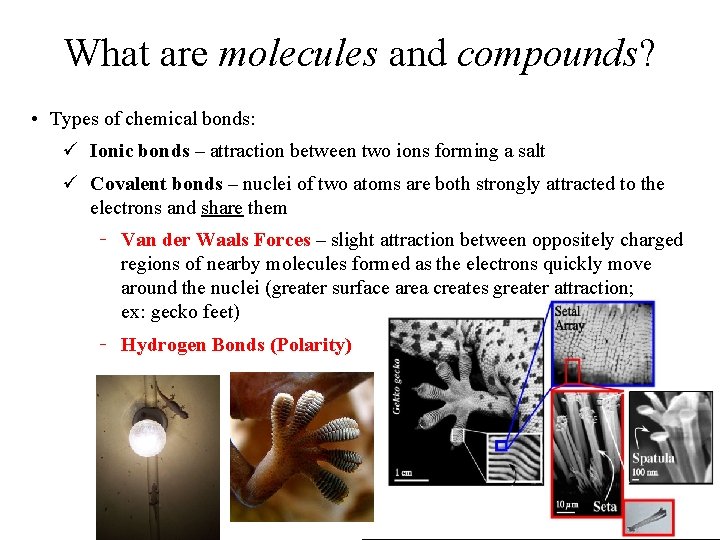
What are molecules and compounds? • Types of chemical bonds: ü Ionic bonds – attraction between two ions forming a salt ü Covalent bonds – nuclei of two atoms are both strongly attracted to the electrons and share them Van der Waals Forces – slight attraction between oppositely charged regions of nearby molecules formed as the electrons quickly move around the nuclei (greater surface area creates greater attraction; ex: gecko feet) Hydrogen Bonds (Polarity)

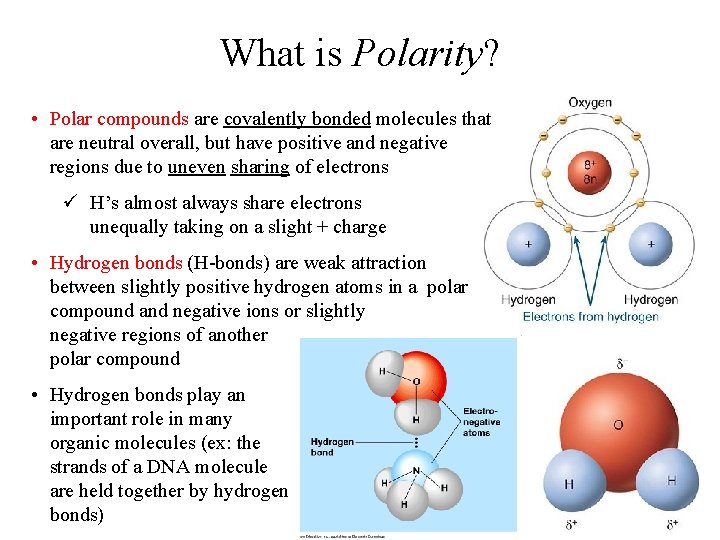
What is Polarity? • Polar compounds are covalently bonded molecules that are neutral overall, but have positive and negative regions due to uneven sharing of electrons ü H’s almost always share electrons unequally taking on a slight + charge • Hydrogen bonds (H-bonds) are weak attraction between slightly positive hydrogen atoms in a polar compound and negative ions or slightly negative regions of another polar compound • Hydrogen bonds play an important role in many organic molecules (ex: the strands of a DNA molecule are held together by hydrogen bonds)

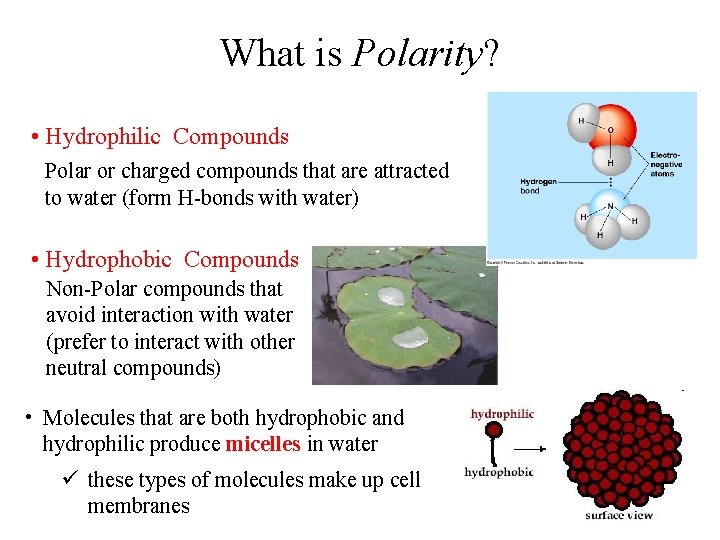
What is Polarity? • Hydrophilic Compounds Polar or charged compounds that are attracted to water (form H-bonds with water) • Hydrophobic Compounds Non-Polar compounds that avoid interaction with water (prefer to interact with other neutral compounds) • Molecules that are both hydrophobic and hydrophilic produce micelles in water ü these types of molecules make up cell membranes

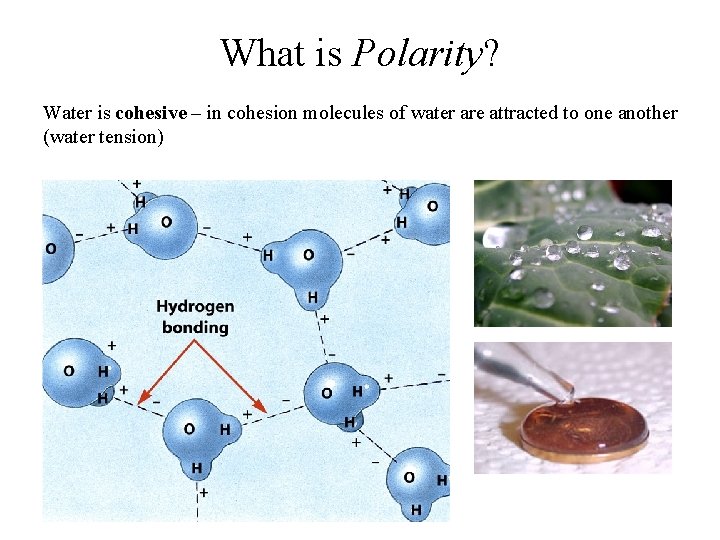
What is Polarity? Water is cohesive – in cohesion molecules of water are attracted to one another (water tension)

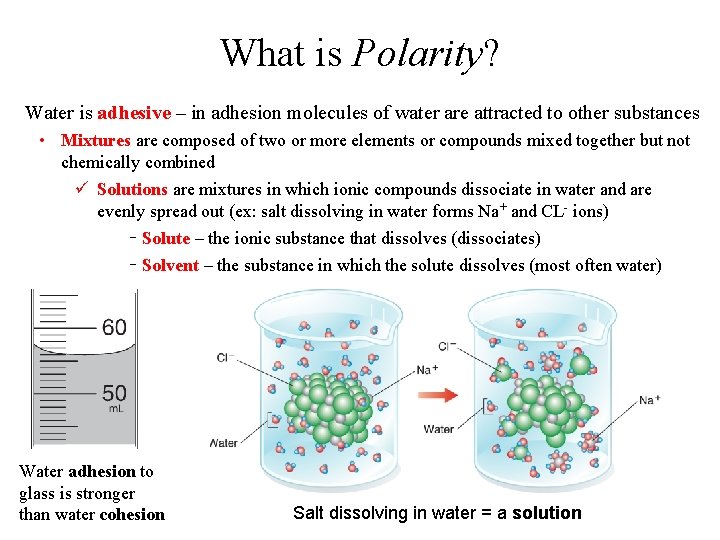
What is Polarity? Water is adhesive – in adhesion molecules of water are attracted to other substances • Mixtures are composed of two or more elements or compounds mixed together but not chemically combined ü Solutions are mixtures in which ionic compounds dissociate in water and are evenly spread out (ex: salt dissolving in water forms Na+ and CL- ions) Solute – the ionic substance that dissolves (dissociates) Solvent – the substance in which the solute dissolves (most often water) Water adhesion to glass is stronger than water cohesion Salt dissolving in water = a solution

What is Polarity? Water is adhesive – in adhesion molecules of water are attracted to other substances • Mixtures are composed of two or more elements or compounds mixed together but not chemically combined ü Solutions are mixtures in which ionic compounds dissociate in water and are evenly spread out (ex: salt dissolving in water forms Na+ and CL- ions) ü Suspensions are mixtures in which tiny clumps of compounds mix with water but DO NOT dissolve/dissociate. Over time, the particles of a suspension may settle to the top or bottom or can be separated out using a filter Ex: fine dirt/sand in water, blood cells in blood ü Mixtures can be separated via Dehydration (water evaporates out) Chromatography (physically separate the particles using some sort of filter – charged materials help separate ions by attracting them) You can separate multiple different particles from a mixture based on size or charge using different types of filters and solutes