Chapter 5 Molecules and Compounds Section 1 Compounds












- Slides: 35

Chapter 5: Molecules and Compounds Section 1: Compounds and Chemical Formulas

Learning Goals �Write chemical formulas. �Determine the total number of each type of atom in a chemical formula. �Classify elements as atomic or molecular. �Classify compounds as ionic or molecular.

Forming Compounds �Sodium is an extremely reactive metal that dulls almost instantly upon exposure to air.

Forming Compounds �Chlorine is a yellow gas with a pungent odor. It is highly reactive and poisonous.

Forming Compounds �The compound formed by sodium and chlorine is table salt. �The properties of a compound are, in general, different from the properties of the elements that compose it.

Forming Compounds �In a compound, the elements combine in fixed, definite proportions. The law of definite proportions (Proust) ▪ Also known as the law of constant composition

Chemical Formulas �A chemical formula indicates the elements present in a compound and the relative number of atoms of each. For example, H 2 O is the chemical formula for water; it indicates that water consists of hydrogen and oxygen atoms in a 2: 1 ratio. �The formula contains the symbol for each element, accompanied by a subscript indicating the number of atoms of that element. By convention, a subscript of 1 is omitted.

Chemical Formulas �What are the element ratios for these common chemical formulas: Na. Cl CO 2 C 12 H 22 O 11

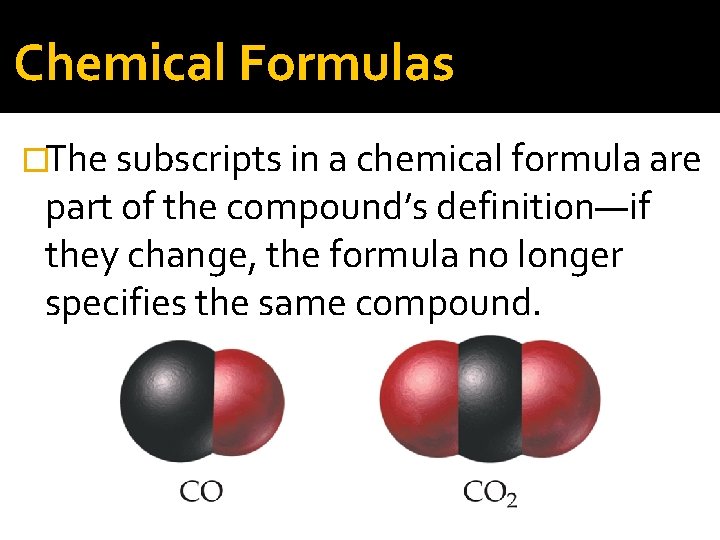
Chemical Formulas �The subscripts in a chemical formula are part of the compound’s definition—if they change, the formula no longer specifies the same compound.

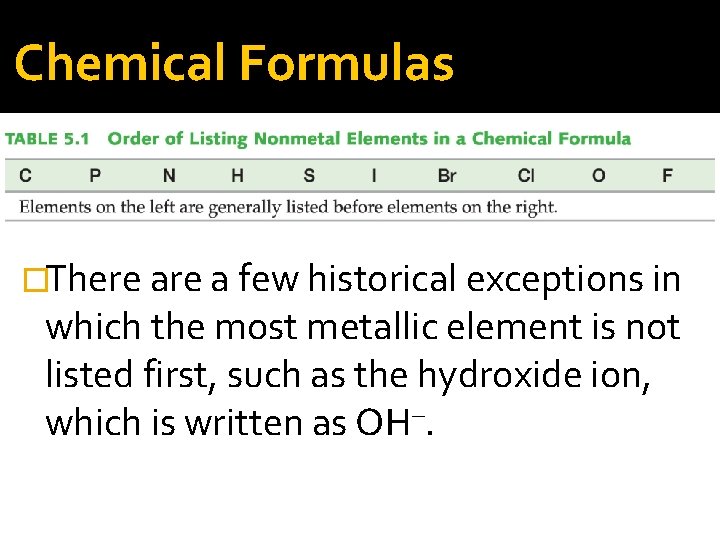
Chemical Formulas �Chemical formulas list the most metallic elements first. The formula for table salt is Na. Cl, not Cl. Na. �In compounds that do not include a metal, the more metal-like element is listed first.

Chemical Formulas �Among nonmetals, those to the left in the periodic table are more metal-like than those to the right and are normally listed first. We write NO 2 and NO, not O 2 N and ON. �Within a single column in the periodic table, elements toward the bottom are more metallike than elements toward the top. We write SO 2, not O 2 S.

Chemical Formulas �There a few historical exceptions in which the most metallic element is not listed first, such as the hydroxide ion, which is written as OH–.

Practice �Write a chemical formula for each compound: The compound containing two aluminum atoms to every three oxygen atoms The compound containing three oxygen atoms to every sulfur atom The compound containing four chlorine atoms to every carbon atom

Polyatomic Ions �Some chemical formulas contain groups of atoms that act as a unit. When several groups of the same kind are present, their formula is set off in parentheses with a subscript to indicate the number of that group. �Mg(NO 3)2 indicates a compound containing one magnesium atom (present as the Mg 2+ ion) and two NO 3– groups.

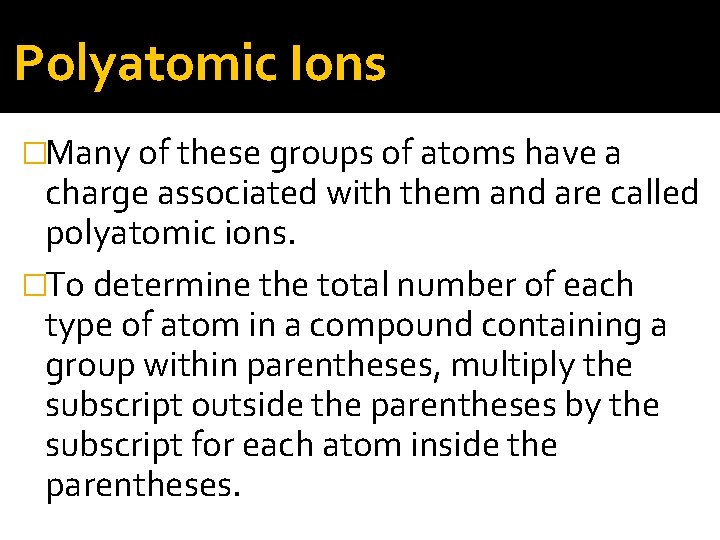
Polyatomic Ions �Many of these groups of atoms have a charge associated with them and are called polyatomic ions. �To determine the total number of each type of atom in a compound containing a group within parentheses, multiply the subscript outside the parentheses by the subscript for each atom inside the parentheses.


Practice �Mg 3(PO 4)2 Mg = ____ P = ____ O = ____

Practice �Al 2(SO 4)3 Al = ____ S = ____ O = ____

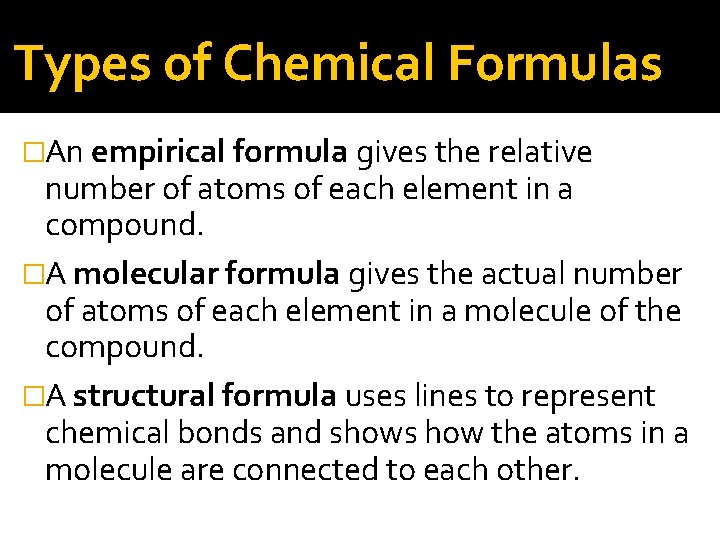
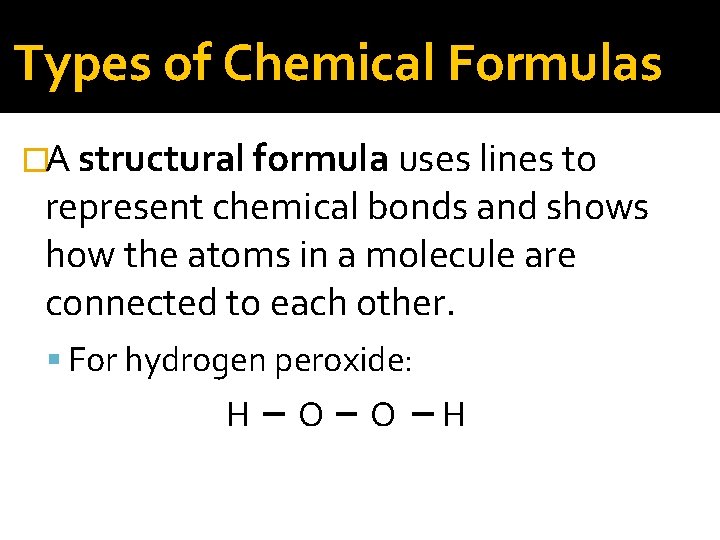
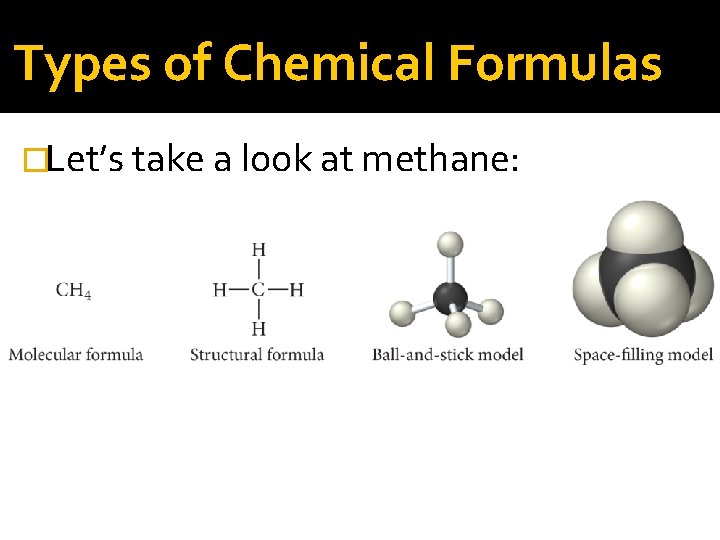
Types of Chemical Formulas �An empirical formula gives the relative number of atoms of each element in a compound. �A molecular formula gives the actual number of atoms of each element in a molecule of the compound. �A structural formula uses lines to represent chemical bonds and shows how the atoms in a molecule are connected to each other.

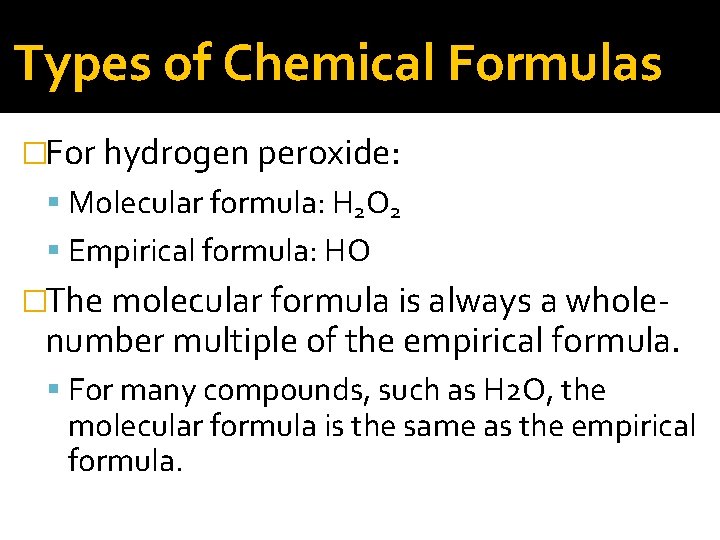
Types of Chemical Formulas �For hydrogen peroxide: Molecular formula: H 2 O 2 Empirical formula: HO �The molecular formula is always a whole- number multiple of the empirical formula. For many compounds, such as H 2 O, the molecular formula is the same as the empirical formula.

Types of Chemical Formulas �A structural formula uses lines to represent chemical bonds and shows how the atoms in a molecule are connected to each other. For hydrogen peroxide: H O O H

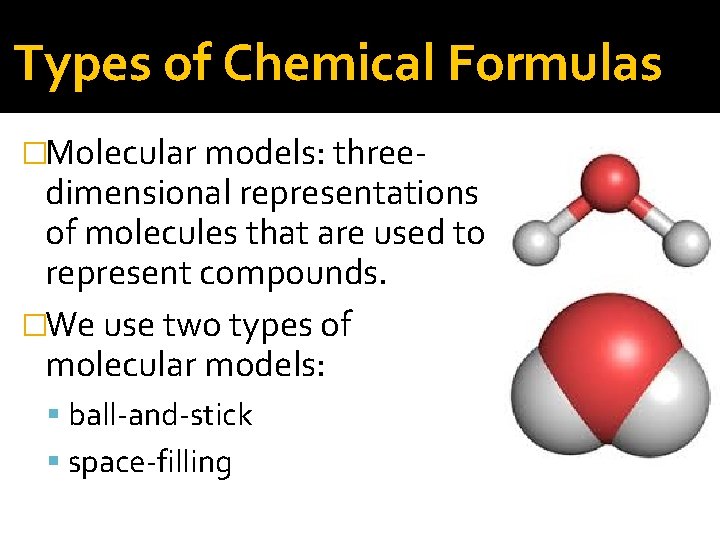
Types of Chemical Formulas �Molecular models: three- dimensional representations of molecules that are used to represent compounds. �We use two types of molecular models: ball-and-stick space-filling

Types of Chemical Formulas �In ball-and-stick models, we represent atoms as balls and chemical bonds as sticks. �The balls and sticks are connected to represent the molecule’s shape. The balls are color coded, and each element is assigned a color.

Types of Chemical Formulas �In space-filing models, atoms fill the space between each other to more closely represent our best idea for how a molecule might appear if we could scale it to a visible size.

Types of Chemical Formulas �Let’s take a look at methane:

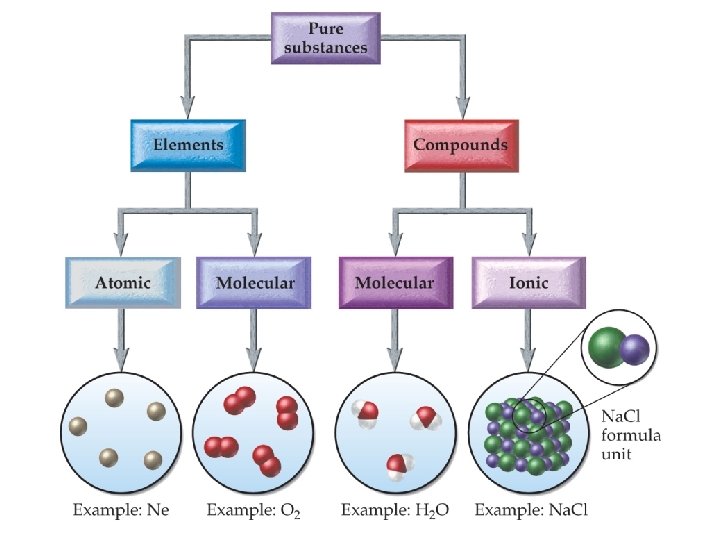
Identifying Substances �Pure substances may be either elements or compounds. �Elements may be either atomic or molecular. �Compounds may be either molecular or ionic.


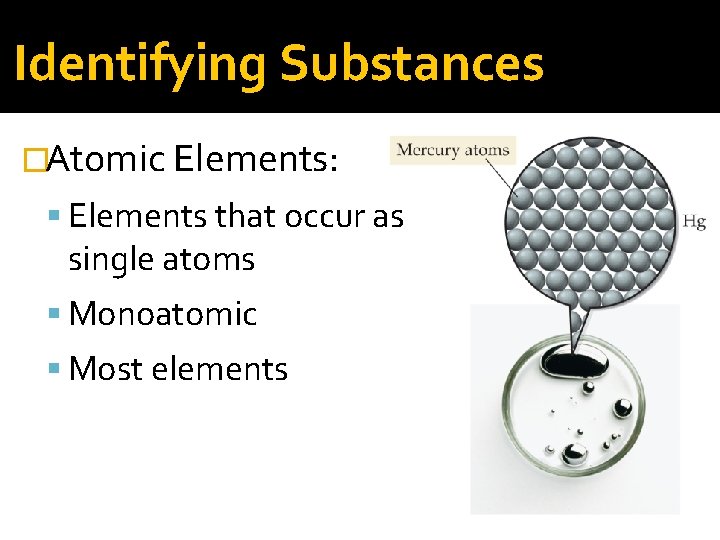
Identifying Substances �Atomic Elements: Elements that occur as single atoms Monoatomic Most elements

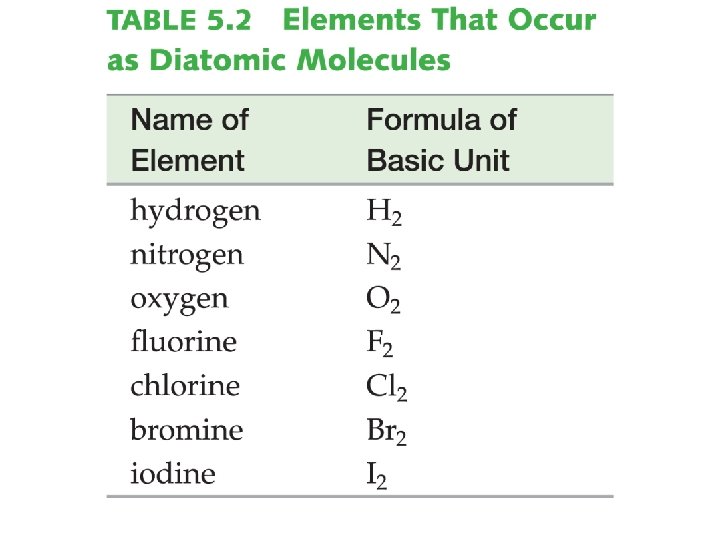
Identifying Substances �Molecular Elements: Elements that occur in pairs Diatomic Seven elements


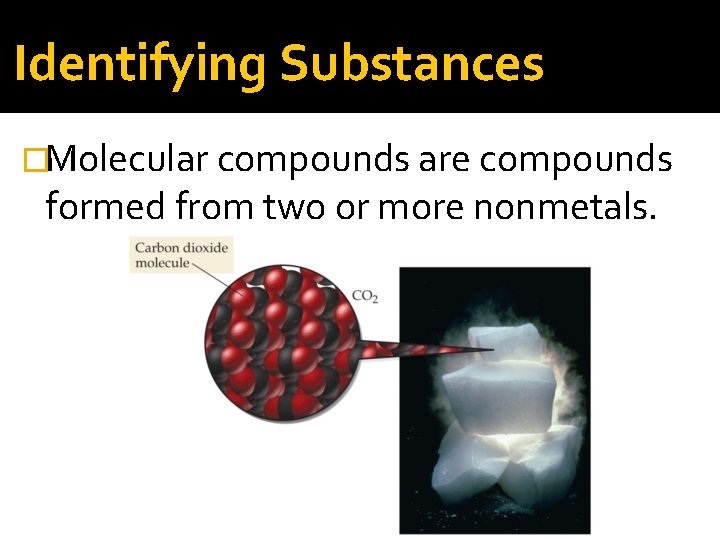
Identifying Substances �Molecular compounds are compounds formed from two or more nonmetals.

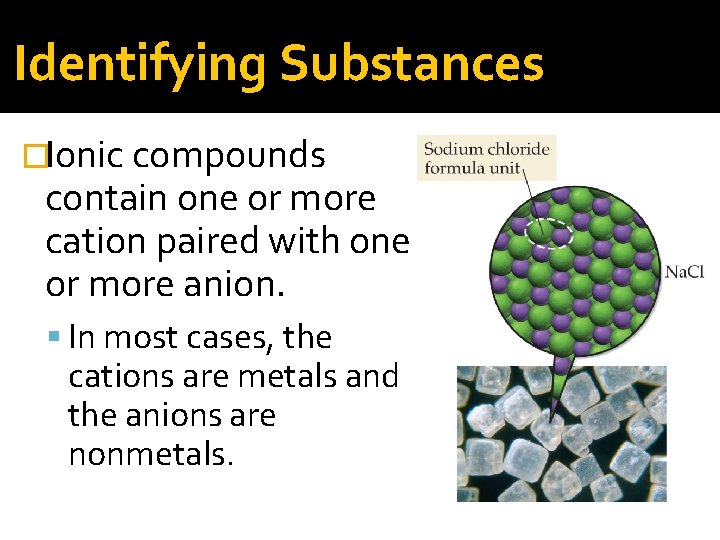
Identifying Substances �Ionic compounds contain one or more cation paired with one or more anion. In most cases, the cations are metals and the anions are nonmetals.

Identifying Substances �When a metal combines with a nonmetal, one or more electrons transfer from the metal to the nonmetal, creating positive and negative ions that are attracted to each other. �A compound composed of a metal and a nonmetal is considered ionic.

Identifying Substances �The basic unit of ionic compounds is the formula unit. �Unlike molecular compounds, ionic compounds do not contain individual molecules but rather cations and anions in an alternating three-dimensional array.

Practice �Classify each substance as an atomic element, molecular element, molecular compound, or ionic compound: Krypton Co. Cl 2 Nitrogen SO 2 KNO 3