Molecules Compounds and Chemical Equations Chapter 3 Compounds

































- Slides: 65

Molecules, Compounds, and Chemical Equations Chapter 3

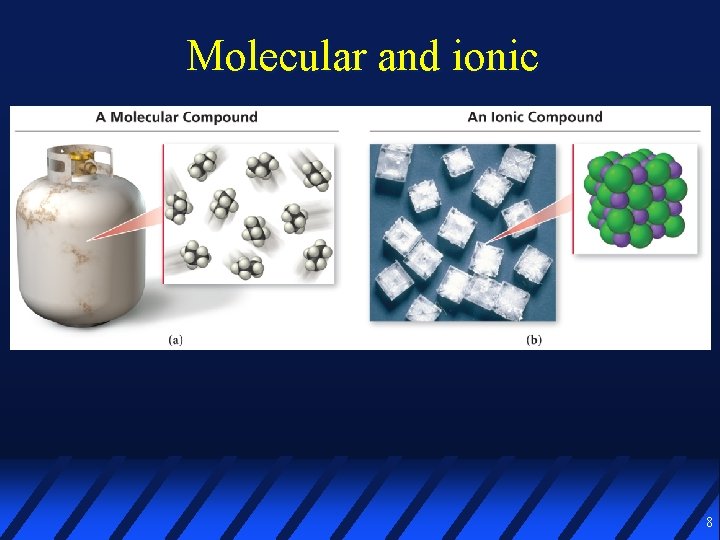
Compounds may be ionic or molecular IONIC COMPOUNDS generally form between metals and nonmetals held together by attraction of oppositely charged ions MOLECULAR COMPOUNDS generally form between nonmetals held together by covalent chemical bonds 2

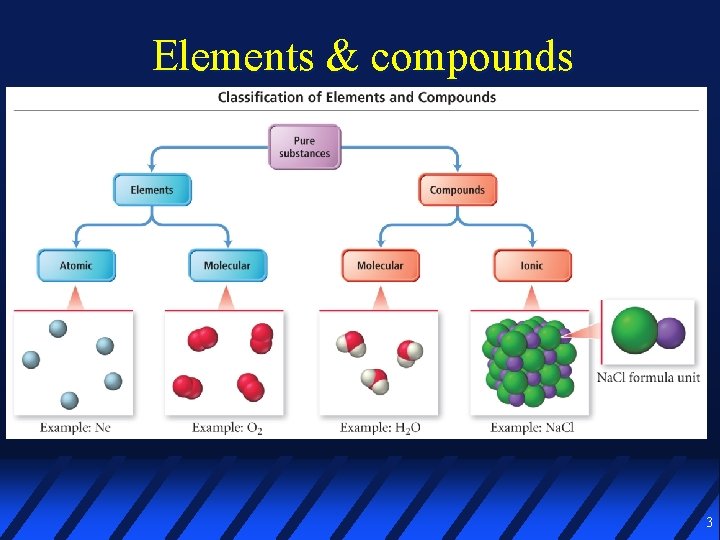
Elements & compounds 3

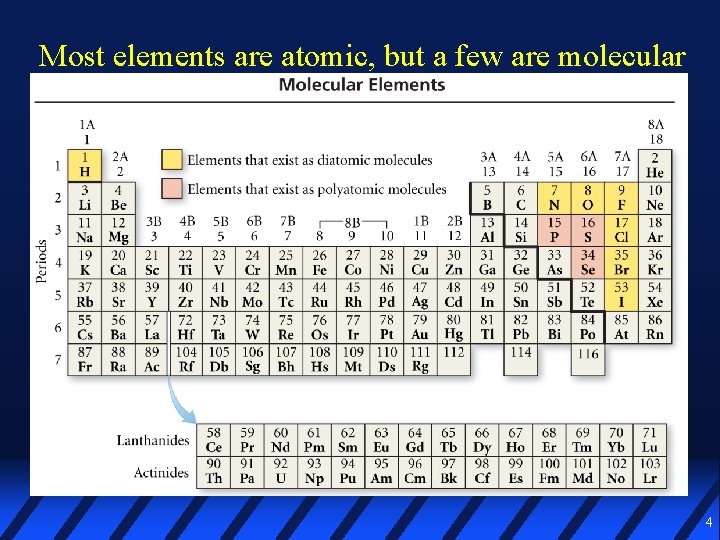
Most elements are atomic, but a few are molecular 4

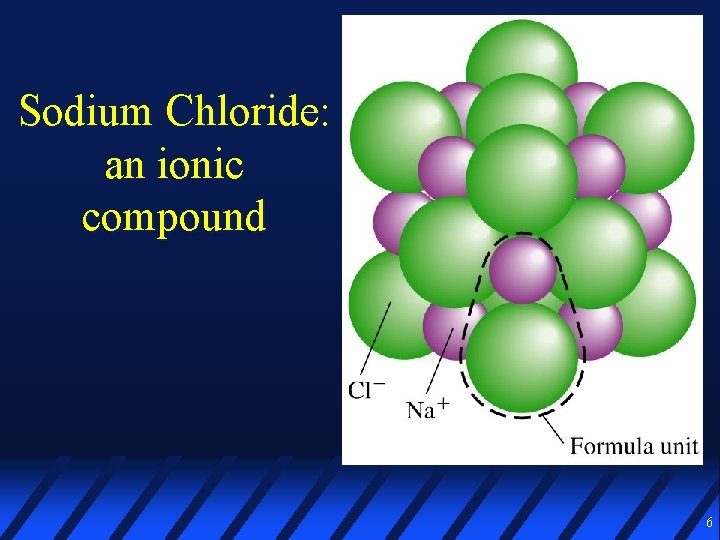
Some compounds are ionic An ionic compound (salt) is made of ions held together by electrostatic forces of attraction Positively charged ions are called cations Negatively charged ions are called anions The basic unit of a salt is a formula unit A formula unit is not a molecule, but the simplest ratio of cations & anions in the lattice 5

Sodium Chloride: an ionic compound 6

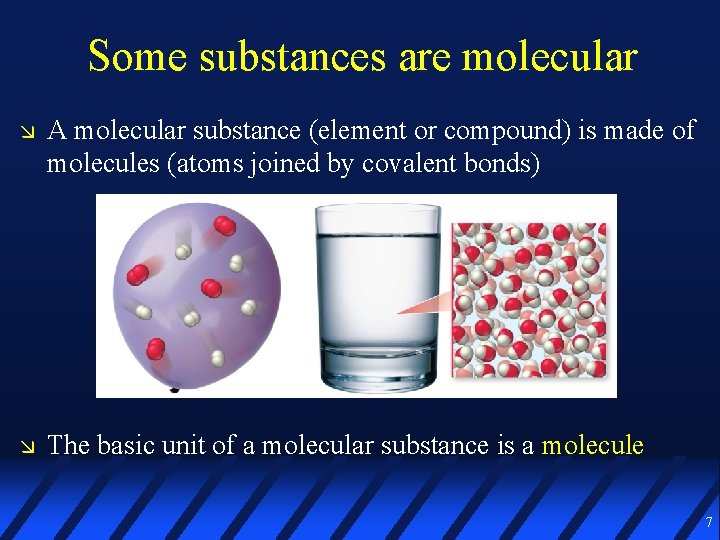
Some substances are molecular A molecular substance (element or compound) is made of molecules (atoms joined by covalent bonds) The basic unit of a molecular substance is a molecule 7

Molecular and ionic 8

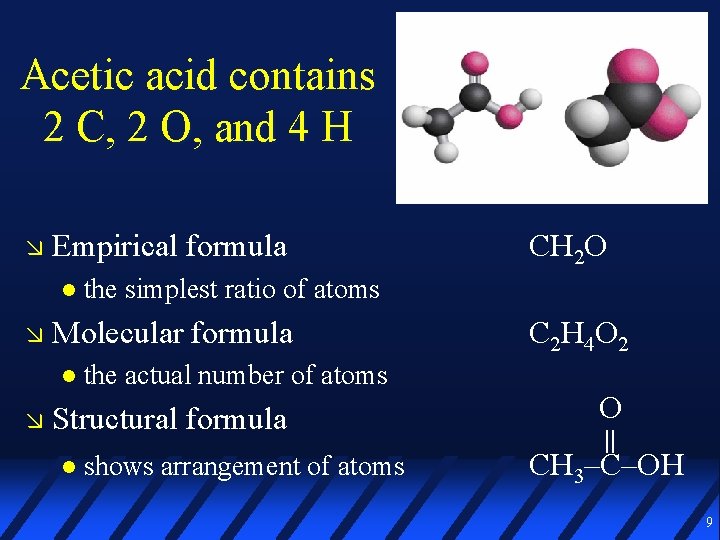
Acetic acid contains 2 C, 2 O, and 4 H Empirical formula the simplest ratio of atoms Molecular formula the actual number of atoms Structural formula CH 2 O shows arrangement of atoms C 2 H 4 O 2 O || CH 3–C–OH 9

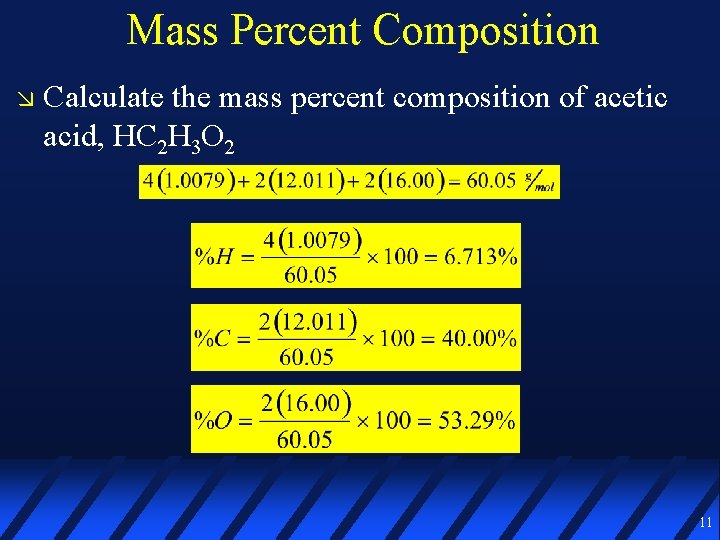
Mass Percent Composition The mass of one element in a compound divided by the total mass of the compound, times 100% 10

Mass Percent Composition Calculate the mass percent composition of acetic acid, HC 2 H 3 O 2 11

Empirical Formula from Percent Composition Use masses given, or assume you have 100 g of compound, so mass of each element = the percent given for each Convert the mass of each element into moles Write a tentative formula based on the moles calculated for each element Divide all subscripts by the smallest value to convert them to small whole numbers 12

Calculating an Empirical Formula Analysis of a sample of a brown gas which is an important air pollutant shows that it contains 2. 34 g of nitrogen and 5. 34 g of oxygen. What is the empirical formula of the gas? 13

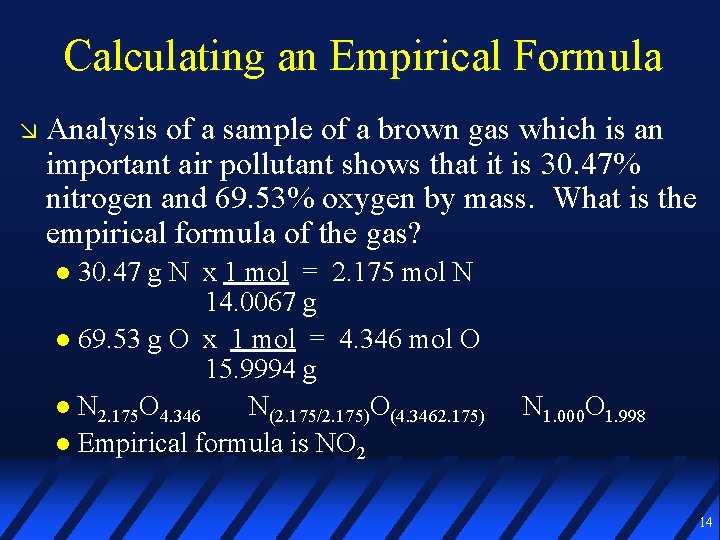
Calculating an Empirical Formula Analysis of a sample of a brown gas which is an important air pollutant shows that it is 30. 47% nitrogen and 69. 53% oxygen by mass. What is the empirical formula of the gas? 30. 47 g N x 1 mol = 2. 175 mol N 14. 0067 g 69. 53 g O x 1 mol = 4. 346 mol O 15. 9994 g N 2. 175 O 4. 346 N(2. 175/2. 175)O(4. 3462. 175) Empirical formula is NO 2 N 1. 000 O 1. 998 14

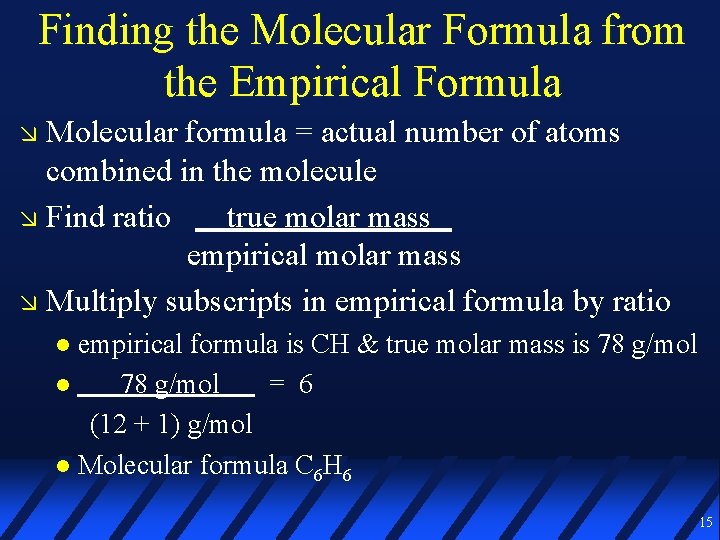
Finding the Molecular Formula from the Empirical Formula Molecular formula = actual number of atoms combined in the molecule Find ratio true molar mass empirical molar mass Multiply subscripts in empirical formula by ratio empirical formula is CH & true molar mass is 78 g/mol = 6 (12 + 1) g/mol Molecular formula C 6 H 6 15

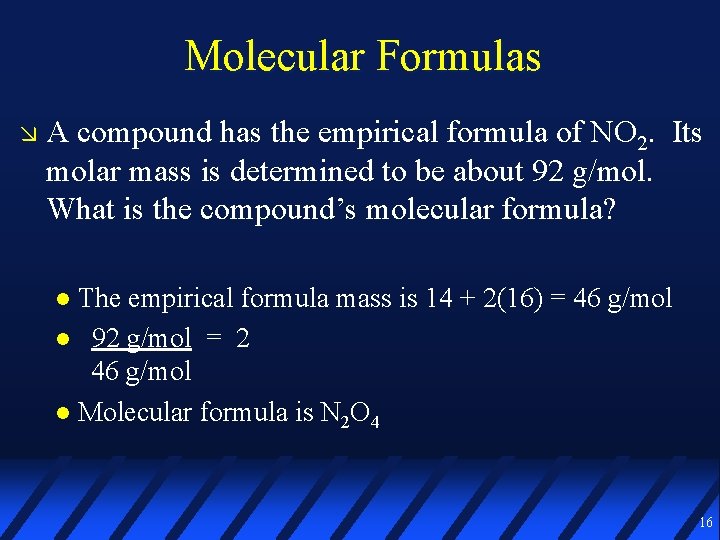
Molecular Formulas A compound has the empirical formula of NO 2. Its molar mass is determined to be about 92 g/mol. What is the compound’s molecular formula? The empirical formula mass is 14 + 2(16) = 46 g/mol 92 g/mol = 2 46 g/mol Molecular formula is N 2 O 4 16

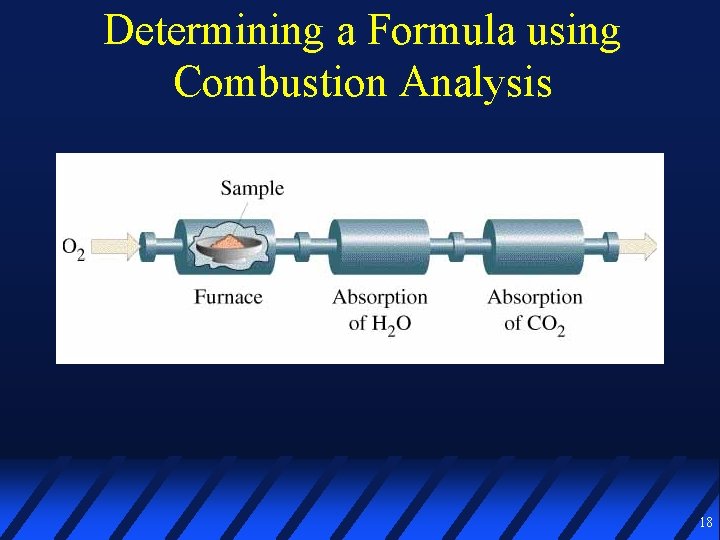
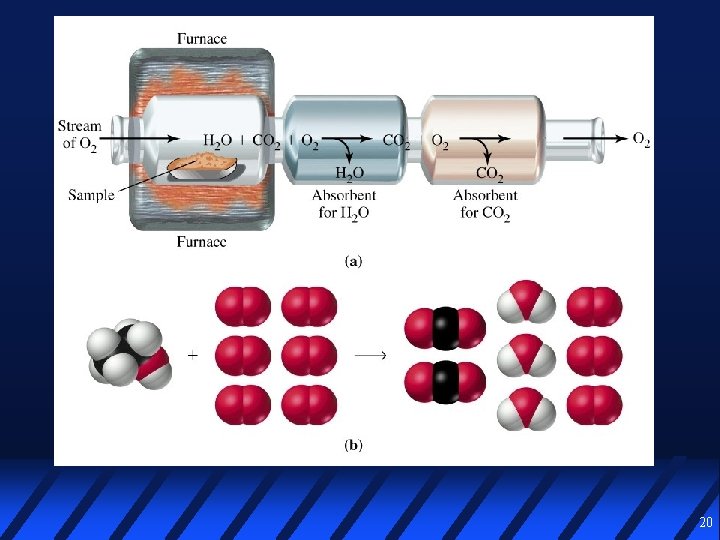
Determining a Formula using Combustion Analysis 18

20

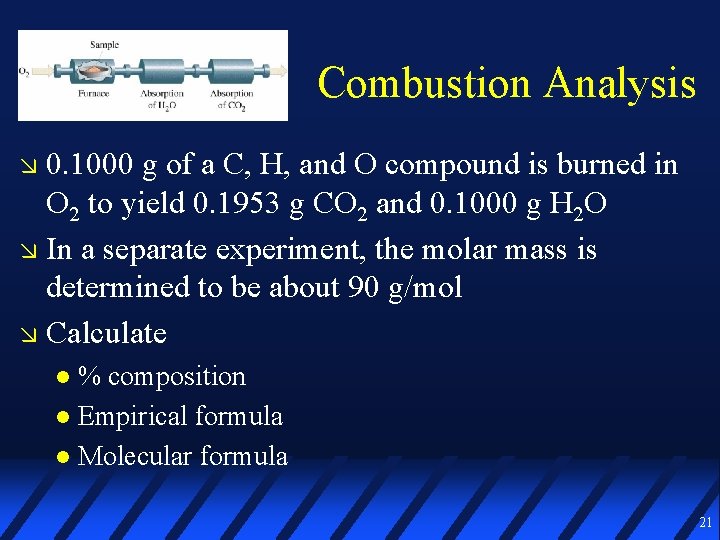
Combustion Analysis 0. 1000 g of a C, H, and O compound is burned in O 2 to yield 0. 1953 g CO 2 and 0. 1000 g H 2 O In a separate experiment, the molar mass is determined to be about 90 g/mol Calculate % composition Empirical formula Molecular formula 21

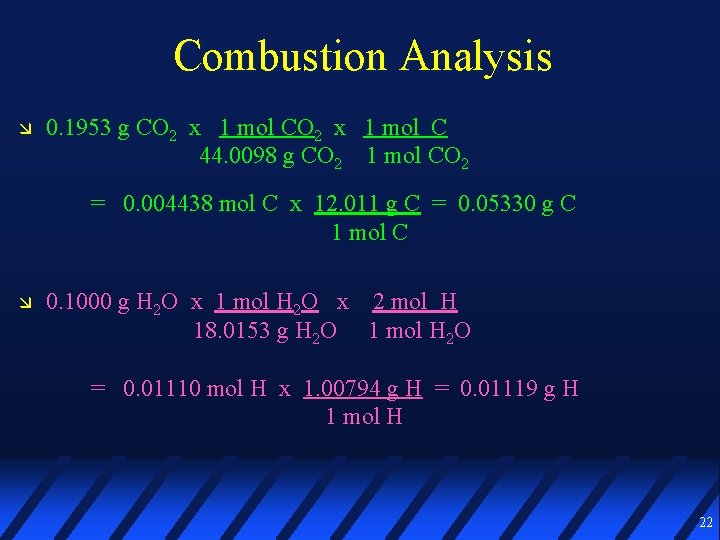
Combustion Analysis 0. 1953 g CO 2 x 1 mol C 44. 0098 g CO 2 1 mol CO 2 = 0. 004438 mol C x 12. 011 g C = 0. 05330 g C 1 mol C 0. 1000 g H 2 O x 1 mol H 2 O x 2 mol H 18. 0153 g H 2 O 1 mol H 2 O = 0. 01110 mol H x 1. 00794 g H = 0. 01119 g H 1 mol H 22

Combustion Analysis 0. 1000 g of the compound contains 0. 05330 g C 0. 01119 g H 0. 06449 g C + H The rest of the compound is oxygen: 0. 1000 g compound – 0. 06449 g C+H = 0. 03551 g O x 1 mol = 0. 002219 mol O 15. 9994 g 23

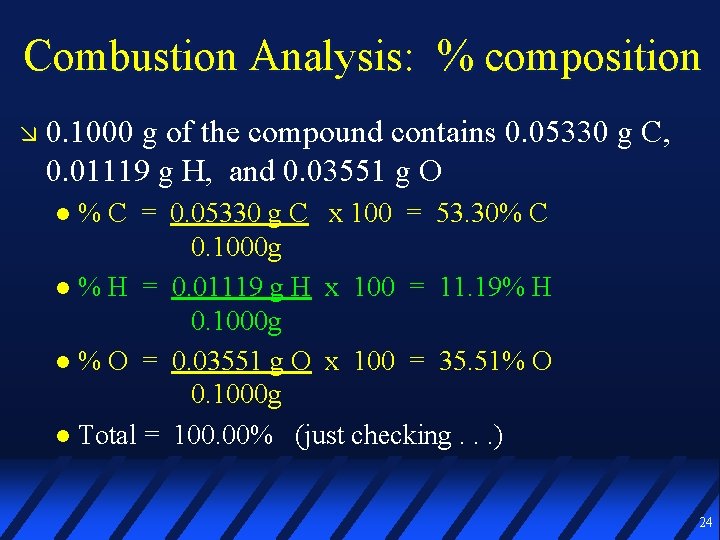
Combustion Analysis: % composition 0. 1000 g of the compound contains 0. 05330 g C, 0. 01119 g H, and 0. 03551 g O % C = 0. 05330 g C x 100 = 53. 30% C 0. 1000 g % H = 0. 01119 g H x 100 = 11. 19% H 0. 1000 g % O = 0. 03551 g O x 100 = 35. 51% O 0. 1000 g Total = 100. 00% (just checking. . . ) 24

Combustion Analysis: empirical formula Compound contains 0. 004438 mol C, 0. 01110 mol H, and 0. 002219 mol O C. 004438 H. 01110 O. 002219 C(. 004438/. 002219)H(. 01110/. 002219)O(. 002219/. 002219) C 2. 000 H 5. 000 O 1. 000 Empirical formula is C 2 H 5 O 25

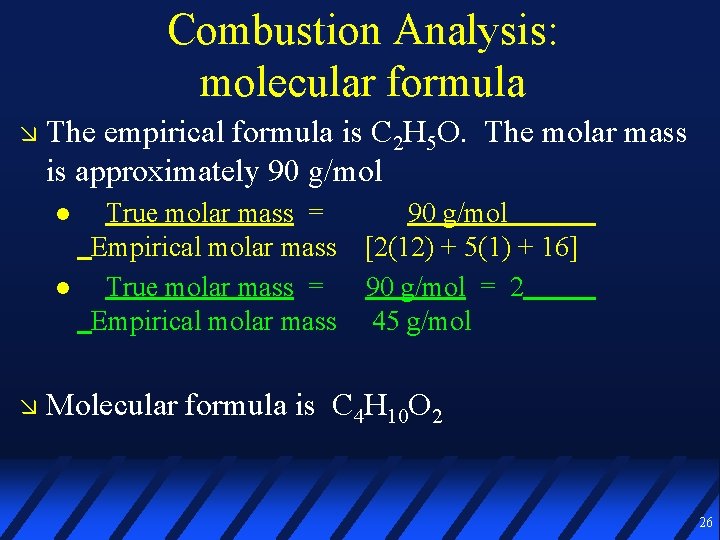
Combustion Analysis: molecular formula The empirical formula is C 2 H 5 O. The molar mass is approximately 90 g/mol True molar mass = Empirical molar mass 90 g/mol [2(12) + 5(1) + 16] 90 g/mol = 2 45 g/mol Molecular formula is C 4 H 10 O 2 26

Oxidation states The oxidation state (O. S. ) or oxidation number is a convenient but artificial way to describe the electron environment around an atom It is related to the number of electrons gained, lost, or apparently used in forming compounds Oxidation states are assigned using the rules on page 79 of your text (memorize these in order) 28

Assigning oxidation states 1. The O. S. of each atom in an element is zero. 2. The total of the O. S. of all atoms in any species (formula unit, molecule or ion) equals the charge on that species. 3. In compounds, Group 1 A metals have O. S. +1 and Group 2 A metals have O. S. +2. 4. In compounds, the O. S. of fluorine is – 1. 5. In compounds, the O. S. of hydrogen is +1. 6. In compounds, the O. S. of oxygen is – 2. 7. In binary compounds with metals, the O. S. of a Group 7 A element is – 1, Group 6 A element – 2, and Group 5 A element – 3. 29

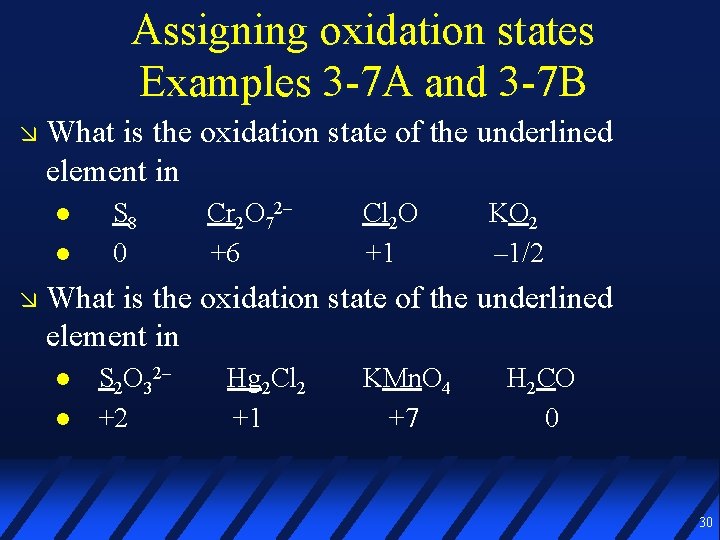
Assigning oxidation states Examples 3 -7 A and 3 -7 B What is the oxidation state of the underlined element in S 8 0 Cr 2 O 72– +6 Cl 2 O +1 KO 2 – 1/2 What is the oxidation state of the underlined element in S 2 O 32– +2 Hg 2 Cl 2 +1 KMn. O 4 +7 H 2 CO 0 30

Nomenclature: Binary Ionic Compounds Binary ionic compounds: metal + nonmetal Nomenclature of binary ionic compounds Write name of metal cation (same as element name) Write name of nonmetal anion, with element name modified to end in “–ide” No prefixes to indicate number of ions Roman numeral shows O. S. of transition metal cation Compound is electrically neutral, so in formula total cation charge = total anion charge 31

Binary ionic compounds Examples 3 -8 A and 3 -8 B Write the formulas for the compounds Lithium oxide Tin (II) fluoride Lithium nitride Write the formulas for the compounds Aluminum sulfide Magnesium nitride Vanadium (III) oxide 32

Binary ionic compounds Examples 3 -8 A and 3 -8 B Write the formulas for the compounds Lithium oxide Tin (II) fluoride Lithium nitride Li 2 O Sn. F 2 Li 3 N Write the formulas for the compounds Aluminum sulfide Magnesium nitride Vanadium (III) oxide Al 2 S 3 Mg 3 N 2 V 2 O 3 33

Binary ionic compounds Examples 3 -9 A and 3 -9 B Write acceptable names for the compounds Cs. I Ca. F 2 Fe. O Cr. Cl 3 Write acceptable names for the compounds Ca. H 2 Cu. Cl Ag 2 S Hg 2 Cl 2 34

Binary ionic compounds Examples 3 -9 A and 3 -9 B Write acceptable names for the compounds Cs. I Ca. F 2 cesium iodide calcium fluoride Fe. O Cr. Cl 3 iron (II) oxide chromium (III) chloride Write acceptable names for the compounds Ca. H 2 calcium Cu. Cl hydride copper (I) chloride Ag 2 S Hg 2 Cl 2 silver (I) sulfide mecury (I) chloride 35

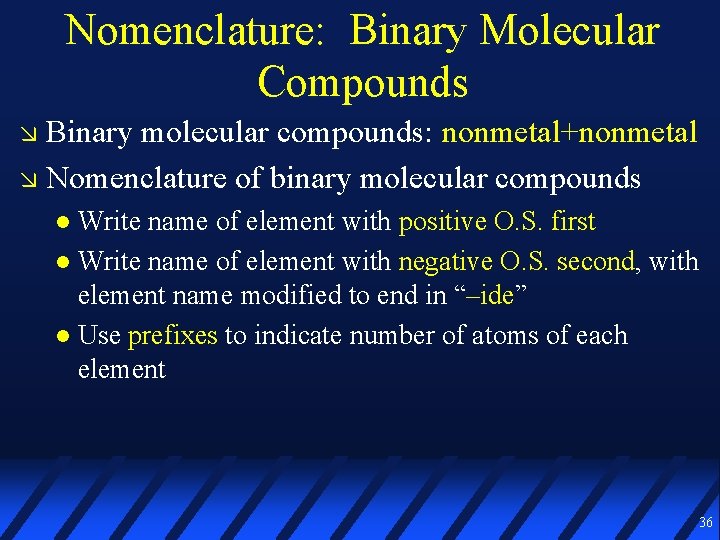
Nomenclature: Binary Molecular Compounds Binary molecular compounds: nonmetal+nonmetal Nomenclature of binary molecular compounds Write name of element with positive O. S. first Write name of element with negative O. S. second, with element name modified to end in “–ide” Use prefixes to indicate number of atoms of each element 36

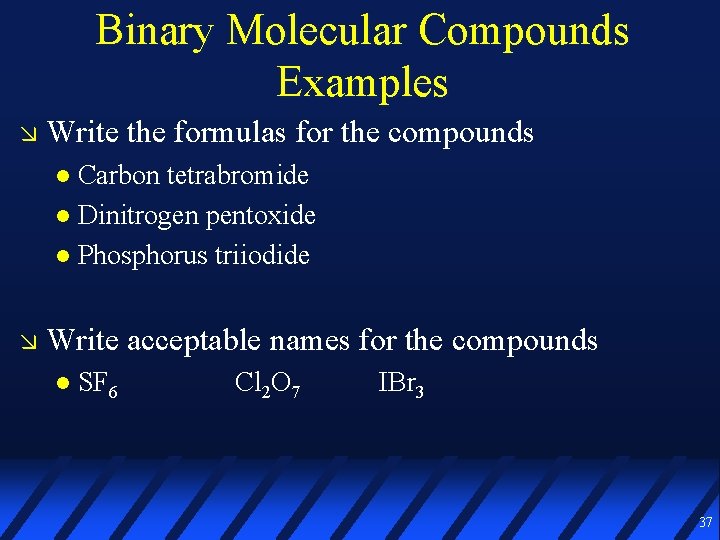
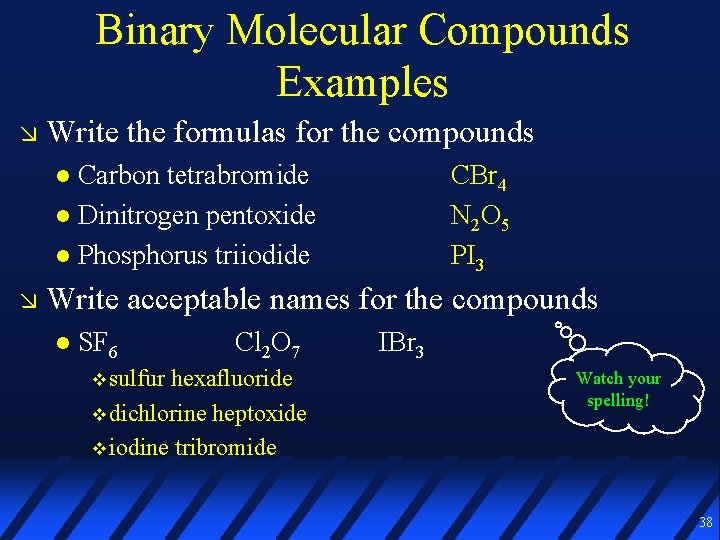
Binary Molecular Compounds Examples Write the formulas for the compounds Carbon tetrabromide Dinitrogen pentoxide Phosphorus triiodide Write acceptable names for the compounds SF 6 Cl 2 O 7 IBr 3 37

Binary Molecular Compounds Examples Write the formulas for the compounds Carbon tetrabromide Dinitrogen pentoxide Phosphorus triiodide CBr 4 N 2 O 5 PI 3 Write acceptable names for the compounds SF 6 sulfur Cl 2 O 7 hexafluoride dichlorine heptoxide iodine tribromide IBr 3 Watch your spelling! 38

Nomenclature: Binary acids: certain compounds of H + nonmetal Produce hydrogen ions (H 1+) when dissolved in water Name as acid when focus is on behavior in water Write prefix “hydro” for hydrogen Write nonmetal element, with name modified to end in “–ic, ” then write acid Formula must be electrically neutral 39

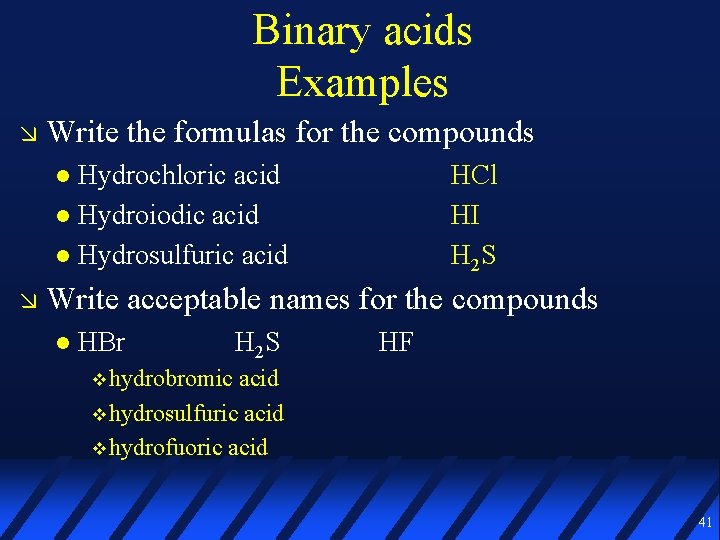
Binary acids Examples Write the formulas for the compounds Hydrochloric acid Hydroiodic acid Hydrosulfuric acid Write acceptable names for the compounds HBr H 2 S HF 40

Binary acids Examples Write the formulas for the compounds Hydrochloric acid Hydroiodic acid Hydrosulfuric acid HCl HI H 2 S Write acceptable names for the compounds HBr H 2 S HF hydrobromic acid hydrosulfuric acid hydrofuoric acid 41

Nomenclature: Polyatomic Ions Polyatomic ions are ions consisting of two or more covalently bound atoms that carry a charge Most are anions (exception: ammonium, NH 41+) Very few polyatomic anion names end in “–ide” Cyanide, CN 1– Hydroxide, OH 1– Most names end in “–ate” or “–ite” 42

Nomenclature: Oxoanions Polyatomic ions that contain oxygen are oxoanions A nonmetal may form several oxoanions with different numbers of oxygens As nonmetal O. S. increases (number of oxygens increases), name changes sysematically: Cl. O 1– Cl. O 21– Cl. O 31– Cl. O 41– hypochlorite chlorate perchlorate SO 32– SO 42– sulfite sulfate All common oxoanions of halogens are – 1 43

Nomenclature: Oxoanions Polyatomic ions that contain oxygen are oxoanions Some oxoanions include varying numbers of hydrogens PO 43– HPO 42– H 2 PO 41– phosphate hydrogen phosphate dihydrogen phosphate O. S. of central nonmetal is constant: ion charge changes as number of hydrogens varies Prefix “thio–” indicates an S has substituted for an O SO 42– sulfate S 2 O 32– thiosulfate 44

Nomenclature: Oxoacids are combinations of hydrogen ions (H 1+) and oxoanions H 1+ + oxoanion = acid, a molecular compound Metal ion + oxoanion = salt, an ionic compound Oxoacid name derived from oxoanion name Change “–ite” to “–ous” and “–ate” to “–ic” Add “acid” to end of name Formulas are electrically neutral 45

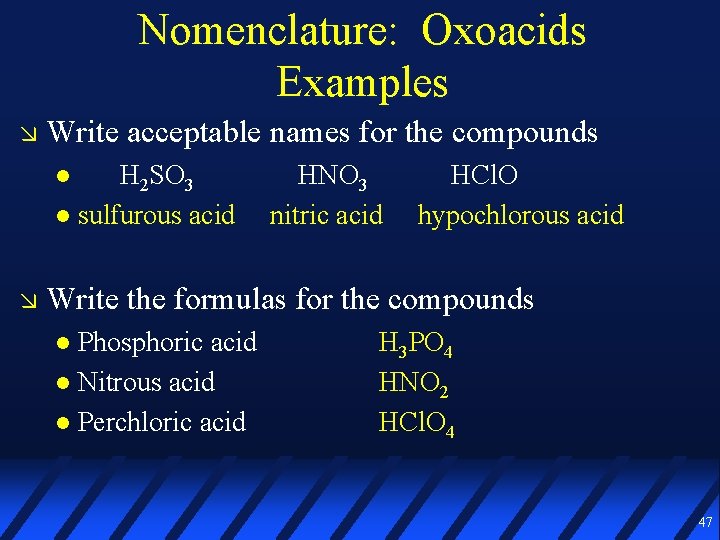
Nomenclature: Oxoacids Examples Write acceptable names for the compounds H 2 SO 3 HNO 3 HCl. O Write the formulas for the compounds Phosphoric acid Nitrous acid Perchloric acid 46

Nomenclature: Oxoacids Examples Write acceptable names for the compounds H 2 SO 3 sulfurous acid HNO 3 nitric acid HCl. O hypochlorous acid Write the formulas for the compounds Phosphoric acid Nitrous acid Perchloric acid H 3 PO 4 HNO 2 HCl. O 4 47

Nomenclature Examples 3 -10 A and 3 -10 B Name the compounds SF 6 NH 4 NO 3 HNO 2 PCl 3 Ca(HCO 3)2 HBr. O Fe. SO 4 Ag. Cl. O 4 Fe 2(SO 4)3 48

Nomenclature Examples 3 -10 A and 3 -10 B Name the compounds SF 6 HNO 2 Ca(HCO 3)2 Sulfur hexafluoride Nitrous acid NH 4 NO 3 PCl 3 Fe. SO 4 Calcium hydrogen carbonate Iron (II) sulfate HBr. O Ag. Cl. O 4 Fe 2(SO 4)3 Ammonium nitrate Phosphorus trichloride Hypobromous acid Ailver perchlorate Iron (III) sulfate 49

Nomenclature Examples 3 -11 A and 3 -11 B Write formulas for the compounds Boron trifluoride Potassium dichromate Sulfuric acid Calcium chloride Aluminum nitrate Tetraphosphorous decoxide Chromium (III) hydroxide Iodic acid 50

Nomenclature Examples 3 -11 A and 3 -11 B Write formulas for the compounds Boron trifluoride Potassium dichromate Sulfuric acid Calcium chloride Aluminum nitrate Tetraphosphorous decoxide Chromium (III) hydroxide Iodic acid BF 3 K 2 Cr 2 O 7 H 2 SO 4 Ca. Cl 2 Al(NO 3)3 P 4 O 10 Cr(OH)3 HIO 3 51

Slaying the nomenclature dragon Make flash cards of all the ion names, formulas and charges and all the acid names and formulas (Tables 3. 1, 3. 3, and 3. 4 in Chapter 3), and of the Greek prefixes (mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca) Concentrate on writing formulas from names (that’s what’s on the AP exam) For ionic formulas and acid, be sure charges balance! 52

Exercise 32 The hemoglobin content of blood is about 15. 5 g/100 m. L blood. The molar mass of hemoglobin is about 64, 500 g/mol, and there are 4 Fe atoms in a hemoglobin molecule. Approximately how many Fe atoms are present in the 6 L of blood in a typical adult? 53

Exercise 38 Determine the mass percent of each element in the antimalarial drug quinine, C 20 H 24 N 2 O 2 54

Exercise 45 Determin the empirical formula of Warfarin, 74. 01% C, 5. 23% H, and 20. 76% O by mass Mustard gas, 30. 20% C, 5. 07% H, 44. 58% Cl, and 20. 16% S by mass 55

Exercise 47 Indigo has the mass composition 73. 27% C, 3. 84% H, 10. 68% N, and the remainder oxygen. Its molecular mass is 262. 3 amu. What is the molecular formula of indigo? 56

Exercise 50 The element X forms the chloride XCl 4 containing 75. 0% Cl, by mass. What is the identity of element X? 57

Exercise 51 A 0. 2612 g sample of a hydrocarbon produces 0. 8661 g CO 2 and 0. 2216 g H 2 O in combustion analysis. Its molecular mass is found to be 106 amu. Determine the mass composition, empirical formula, and molecular formula of this hydrocarbon. 58

Exercises 57 and 60 Indicate the oxidations state of the underlined element: CH 4 SF 4 Na 2 O 2 C 2 H 3 O 21– Fe. O 42– Nitrogen forms five oxides. Write appropriate formulas for these compounds if the O. S. of N in them are +1, +2, +3, +4, and +5, respectively. 59

Exercise 62 Name these compounds Ba(NO 3)2 HNO 2 Cr. O 2 KIO 3 Li. CN KIO Fe(OH)2 Ca(H 2 PO 4)2 H 3 PO 4 Na. HSO 4 Na 2 Cr 2 O 7 NH 4 C 2 H 3 O 2 Mg. C 2 O 4 Na 2 C 2 O 4 60

Exercise 64 Assign suitable names to these compounds ICl Cl. F 3 SF 4 Br. F 5 N 2 O 4 S 4 N 4 61

Exercise 66 Write correct formulas for Magnesium perchlorate Lead (II) acetate Tin (IV) oxide Hydroiodic acid Chlorous acid Sodium hydrogen sulfite Calcium dihydrogen phosphate Aluminum phosphate Dinitrogen tetroxide Disulfur dichloride 62

Exercise 77 A sample of Mg. SO 4 • x. H 2 O weighing 8. 129 g is heated until all the water of hydration is driven off. The resulting anhydrous compound, Mg. SO 4, weighs 3. 967 g. What is the formula of the hydrate? 63

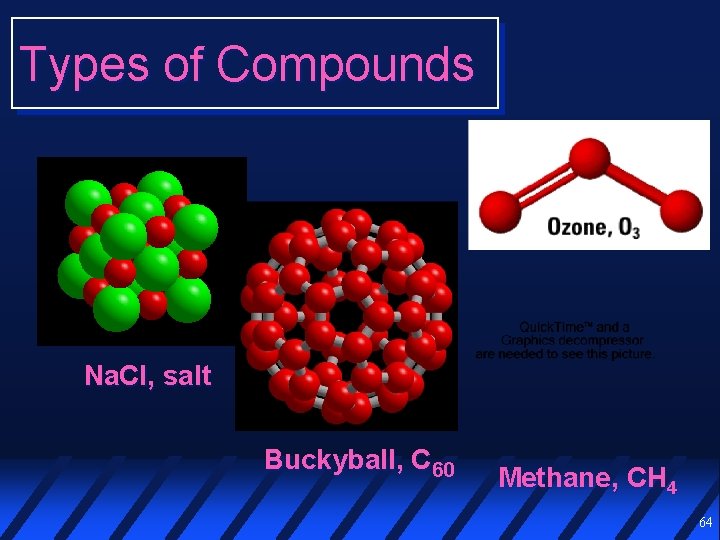
Types of Compounds Na. Cl, salt Buckyball, C 60 Methane, CH 4 64

Molecular Compounds A molecule is a group of bonded atoms that exists as a distinct entity The atoms in a molecule are held together by covalent bonds Molecular compounds consist of discrete molecules 70

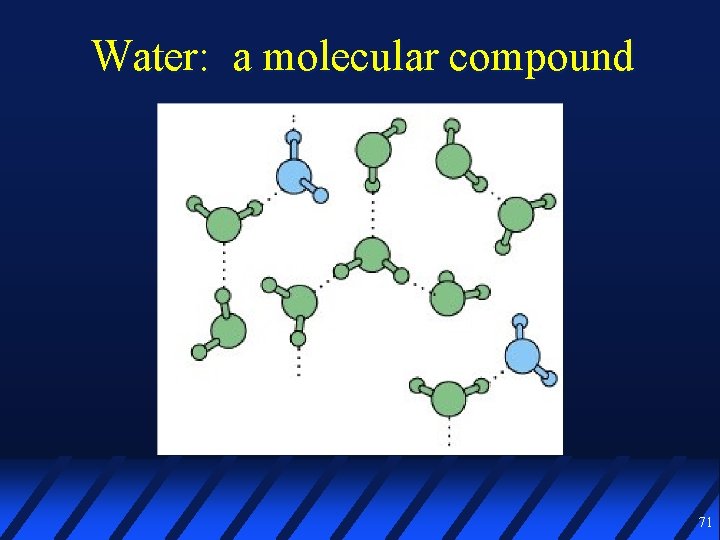
Water: a molecular compound 71

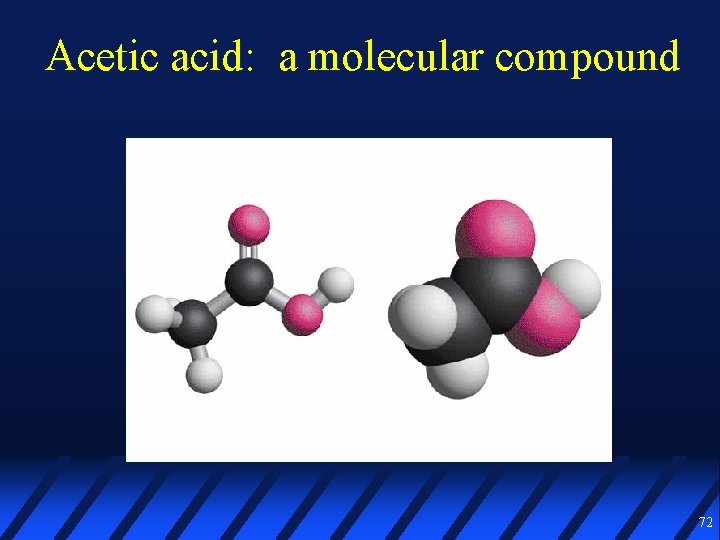
Acetic acid: a molecular compound 72

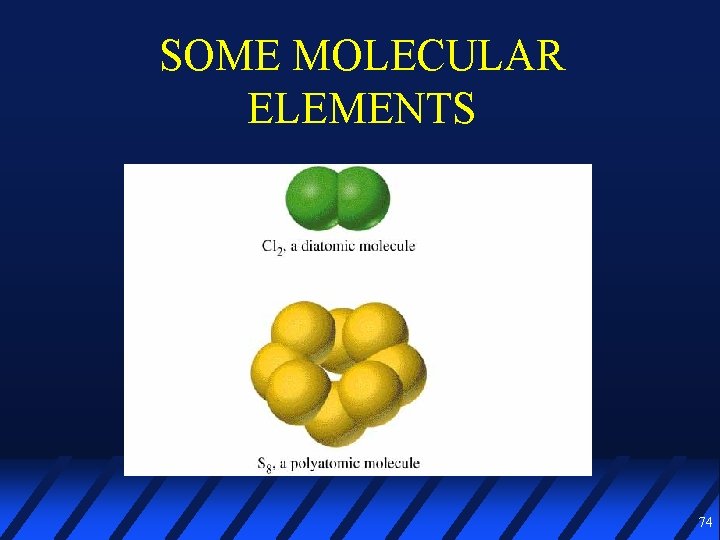
SOME MOLECULAR ELEMENTS 74