Molecular Compounds Molecules and Molecular Compounds atoms held






- Slides: 12

Molecular Compounds

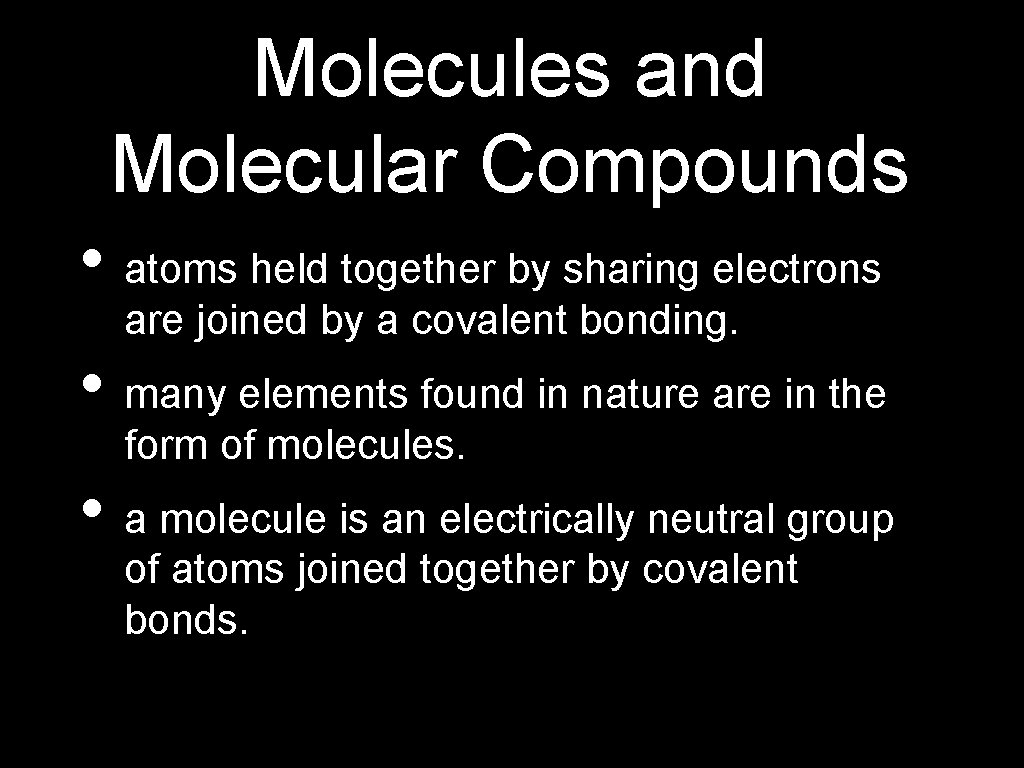
Molecules and Molecular Compounds • atoms held together by sharing electrons are joined by a covalent bonding. • many elements found in nature are in the form of molecules. • a molecule is an electrically neutral group of atoms joined together by covalent bonds.

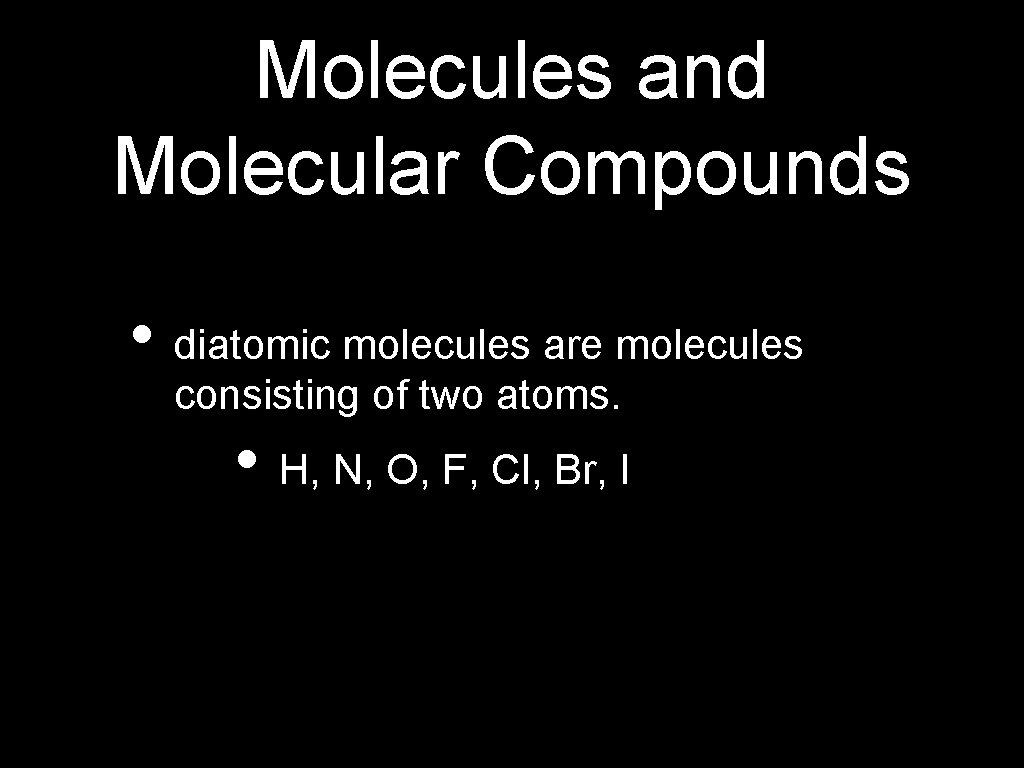
Molecules and Molecular Compounds • diatomic molecules are molecules consisting of two atoms. • H, N, O, F, Cl, Br, I

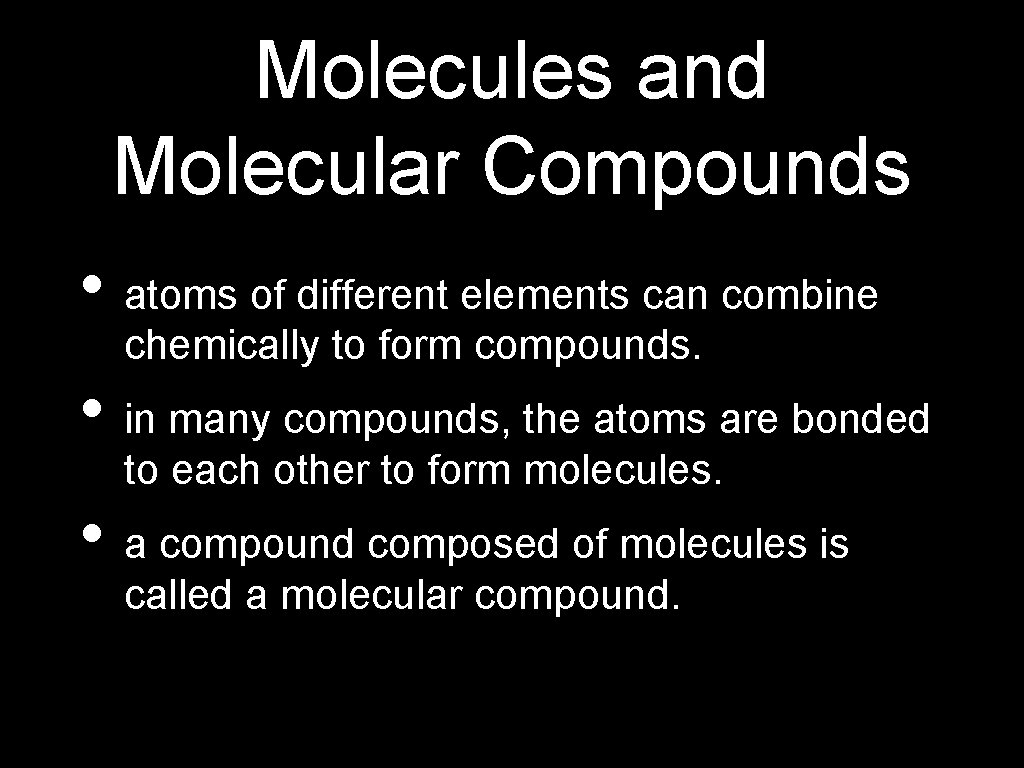
Molecules and Molecular Compounds • atoms of different elements can combine chemically to form compounds. • in many compounds, the atoms are bonded to each other to form molecules. • a compound composed of molecules is called a molecular compound.

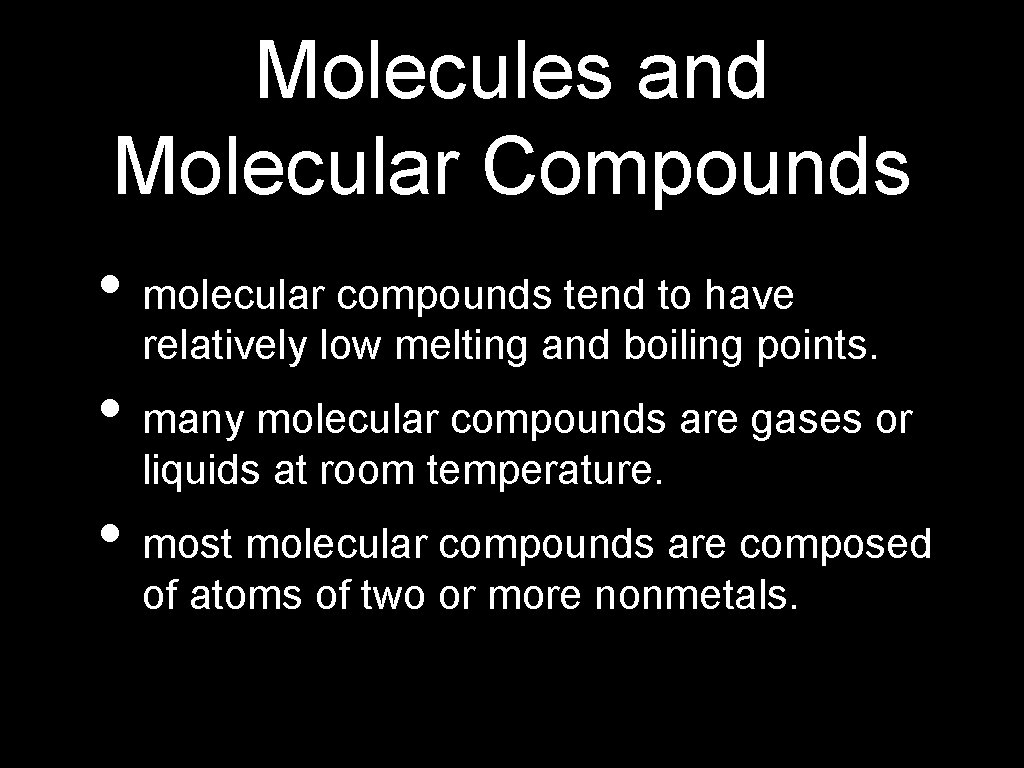
Molecules and Molecular Compounds • molecular compounds tend to have relatively low melting and boiling points. • many molecular compounds are gases or liquids at room temperature. • most molecular compounds are composed of atoms of two or more nonmetals.

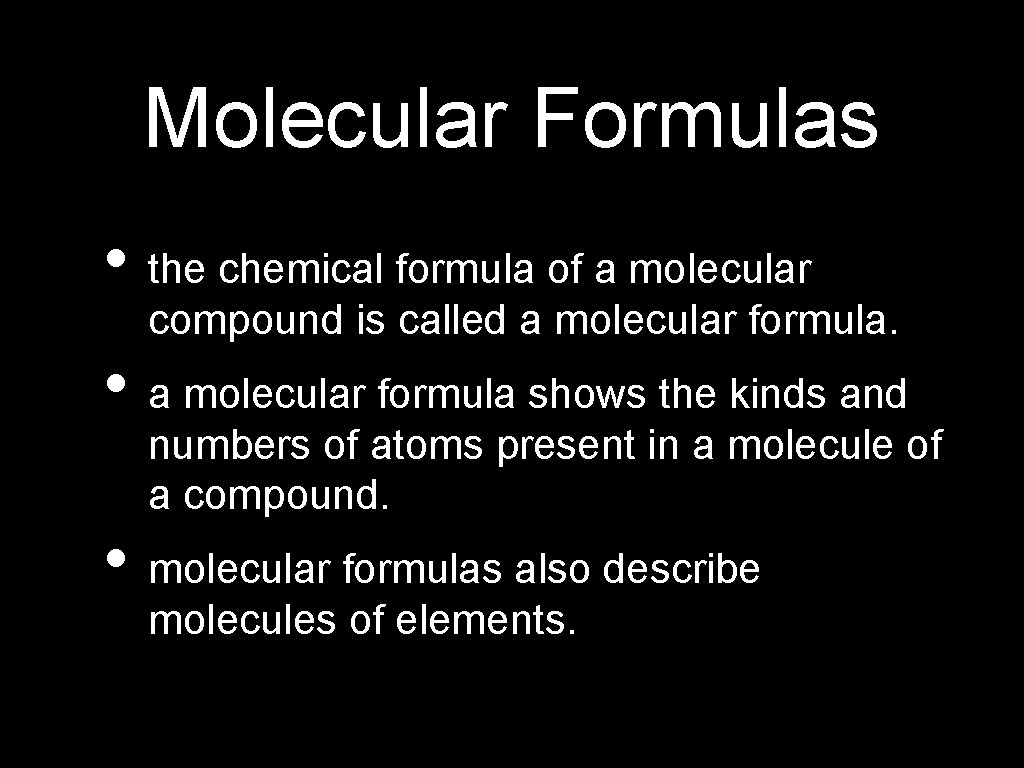
Molecular Formulas • the chemical formula of a molecular compound is called a molecular formula. • a molecular formula shows the kinds and numbers of atoms present in a molecule of a compound. • molecular formulas also describe molecules of elements.

Molecular Formulas • a molecular formula shows how many atoms of each element a molecule contains. • EX: CO - 1 atom of carbon, 2 atoms of oxygen 2

The Nature of Covalent Bonding

The Octet Rule in Covalent Bonding • in forming ionic compounds, electrons tend to be transferred so that each ion acquires a noble gas configuration. • in forming covalent bonds, electron sharing tends to occur so that atoms, with shared electrons included, attain the configurations of noble gases.

Single Covalent Bonds • two atoms held together by sharing a pair of electrons are joined by a single covalent bond. • an electron dot formula such as H: H represents the shared pair of electrons of the covalent bond by two dots. • the pair of shared electrons forming the covalent bond is represented as a dash, H-H

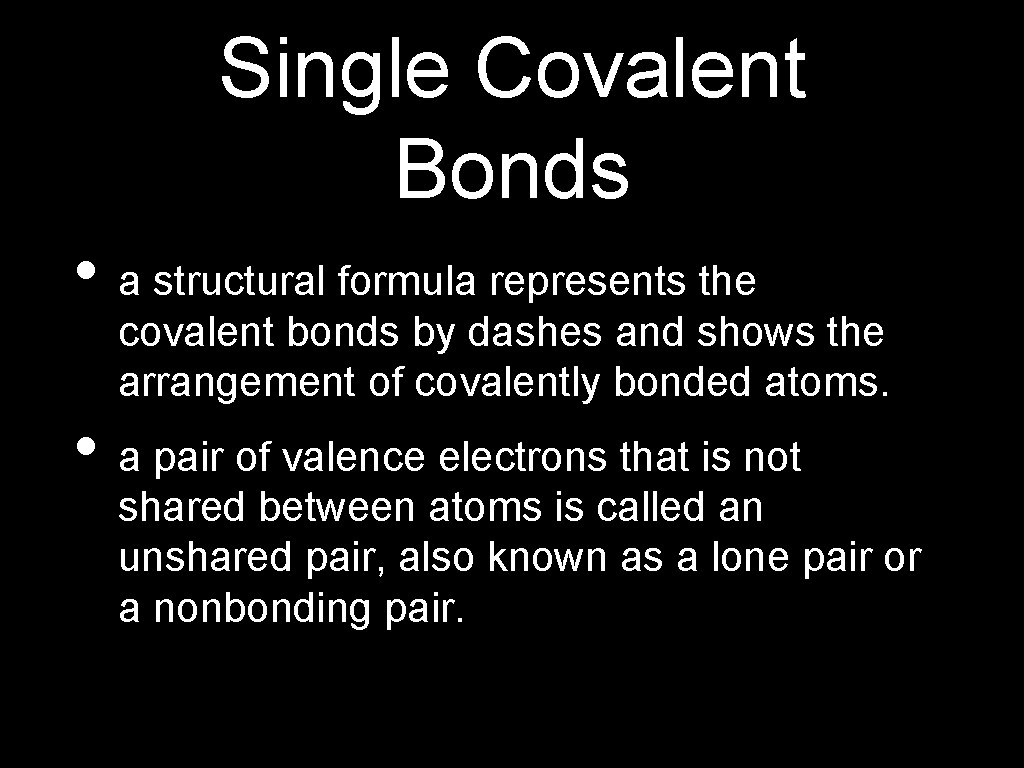
Single Covalent Bonds • a structural formula represents the covalent bonds by dashes and shows the arrangement of covalently bonded atoms. • a pair of valence electrons that is not shared between atoms is called an unshared pair, also known as a lone pair or a nonbonding pair.

Double and Triple Covalent Bonds • atoms form double or triple covalent bonds if they can attain a noble gas structure by sharing two pairs or three pairs of electrons.