VALsartan In Acute myocardial i Nfarc Tion Marc



- Slides: 30

VALsartan In Acute myocardial i. Nfarc. Tion Marc A. Pfeffer, M. D. , Ph. D. (Chair), John J. V. Mc. Murray, M. D. (Co-Chair), Eric J. Velazquez, M. D. , Jean-Lucien Rouleau, M. D. , Lars Køber, M. D. , Aldo P. Maggioni, M. D. , Scott D. Solomon, M. D. , Karl Swedberg, M. D. , Ph. D. , Frans Van de Werf, M. D. , Ph. D. , Harvey D. White, D. Sc. , Jeffrey D. Leimberger, Ph. D. , Marc Henis, M. D. , Susan Edwards, M. S. , Steven Zelenkofske, D. O. , Mary Ann Sellers, M. S. N. , and Robert M. Califf, M. D. , for the VALIANT Investigators Other Steering Committee Members: P. Aylward, P. Armstrong, S. Barvik, Y. Belenkov, A. Dalby, R. Diaz, H. Drexler, G. Ertl, G. Francis, J. Hampton, A. Harsanyi, J. Kvasnicka, V. Mareev, J. Marin-Neto, J. Murin, M. Myers, R. Nordlander, G. Opolski, J. Soler-Soler, J. Spac, T. Stefenelli, D. Sugrue, W. Van Gilst, S. Varshavsky, D. Weaver, F. Zannad. Dr. Pfeffer is named as a coinventor on a patent awarded to the Brigham and Women’s Hospital regarding the use of inhibitors of the renin-angiotensin system in selected survivors of myocardial infarction; there is a licensing agreement between Novartis Pharmaceuticals and the Brigham and Women’s Hospital, which is not linked to sales. Supported by a grant from Novartis Pharmaceuticals

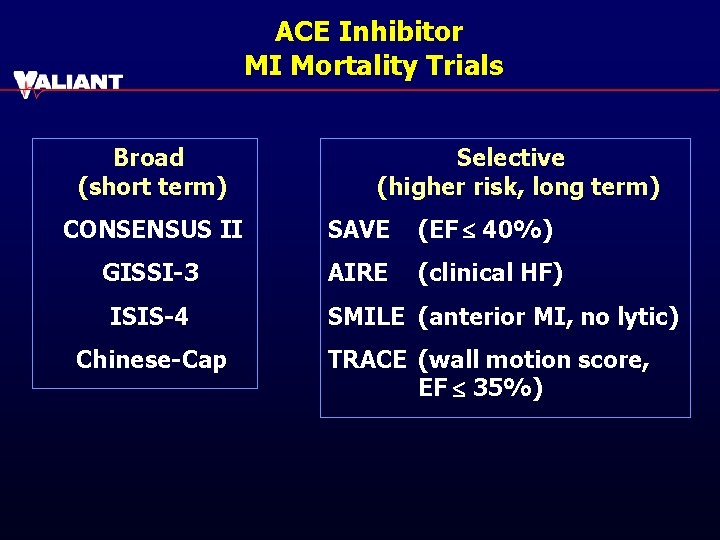
ACE Inhibitor MI Mortality Trials Broad (short term) Selective (higher risk, long term) CONSENSUS II SAVE (EF £ 40%) GISSI-3 AIRE (clinical HF) ISIS-4 Chinese-Cap SMILE (anterior MI, no lytic) TRACE (wall motion score, EF £ 35%)

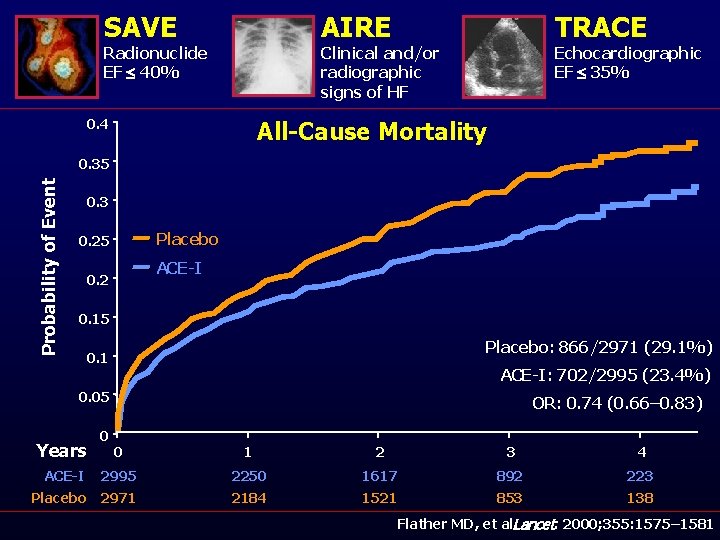
SAVE AIRE Radionuclide EF £ 40% TRACE Clinical and/or radiographic signs of HF 0. 4 Echocardiographic EF £ 35% All-Cause Mortality Probability of Event 0. 35 0. 3 Placebo 0. 25 ACE-I 0. 2 0. 15 Placebo: 866/2971 (29. 1%) 0. 1 ACE-I: 702/2995 (23. 4%) 0. 05 Years 0 OR: 0. 74 (0. 66– 0. 83) 0 1 2 3 4 ACE-I 2995 2250 1617 892 223 Placebo 2971 2184 1521 853 138 Flather MD, et al. Lancet. 2000; 355: 1575– 1581

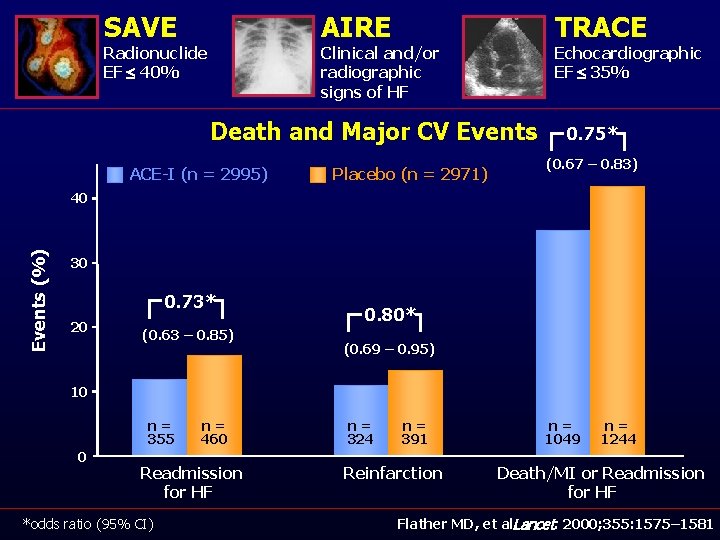
SAVE AIRE Radionuclide EF £ 40% TRACE Clinical and/or radiographic signs of HF Echocardiographic EF £ 35% Death and Major CV Events ACE-I (n = 2995) Placebo (n = 2971) 0. 75* (0. 67 – 0. 83) Events (%) 40 30 0. 73* 20 (0. 63 – 0. 85) 0. 80* (0. 69 – 0. 95) 10 n= 355 0 n= 460 Readmission for HF *odds ratio (95% CI) n= 324 n= 391 Reinfarction n= 1049 n= 1244 Death/MI or Readmission for HF Flather MD, et al. Lancet. 2000; 355: 1575– 1581

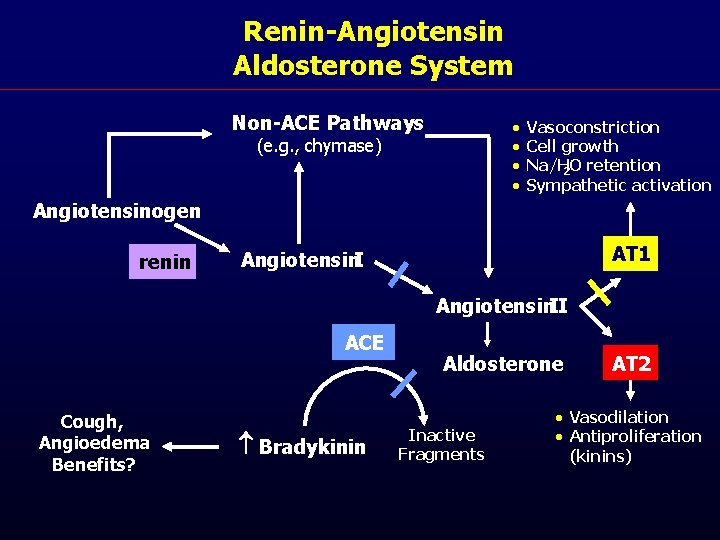
Renin-Angiotensin Aldosterone System Non-ACE Pathways · · (e. g. , chymase) Vasoconstriction Cell growth Na/H 2 O retention Sympathetic activation Angiotensinogen renin AT 1 Angiotensin. II ACE Cough, Angioedema Benefits? Bradykinin Aldosterone Inactive Fragments AT 2 · Vasodilation · Antiproliferation (kinins)

Aims VALIANT was designed as a mortality trial in high-risk MI patients (SAVE, AIRE, TRACE) who derived particular benefits from an ACE inhibitor. To determine whether: u the ARBvalsartanwas superior to captopril in improving surviv and with equal statistical power u the addition of the ARB valsartanto captopril was superior to th proven dose of captopril in improving survival u If valsartanwas not superior to captopril, a non-inferiority analysis was prespecified to determine whether valsartancould be considered “as effective as” captopril

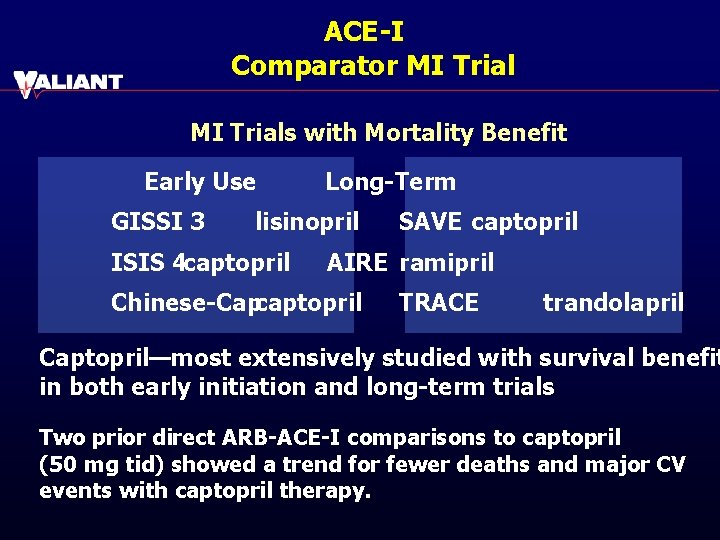
ACE-I Comparator MI Trials with Mortality Benefit Early Use GISSI 3 Long-Term lisinopril ISIS 4 captopril SAVE captopril AIRE ramipril Chinese-Capcaptopril TRACE trandolapril Captopril—most extensively studied with survival benefit in both early initiation and long-term trials Two prior direct ARB-ACE-I comparisons to captopril (50 mg tid) showed a trend for fewer deaths and major CV events with captopril therapy.

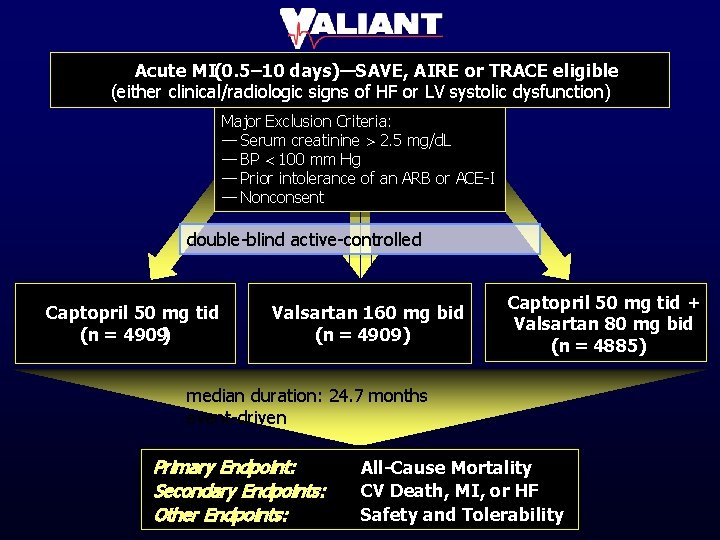
Acute MI(0. 5– 10 days)—SAVE, AIRE or TRACE eligible (either clinical/radiologic signs of HF or LV systolic dysfunction) Major Exclusion Criteria: — Serum creatinine > 2. 5 mg/d. L — BP < 100 mm Hg — Prior intolerance of an ARB or ACE-I — Nonconsent double-blind active-controlled Captopril 50 mg tid (n = 4909) Valsartan 160 mg bid (n = 4909) Captopril 50 mg tid + Valsartan 80 mg bid (n = 4885) median duration: 24. 7 months event-driven Primary Endpoint: Secondary Endpoints: Other Endpoints: All-Cause Mortality CV Death, MI, or HF Safety and Tolerability

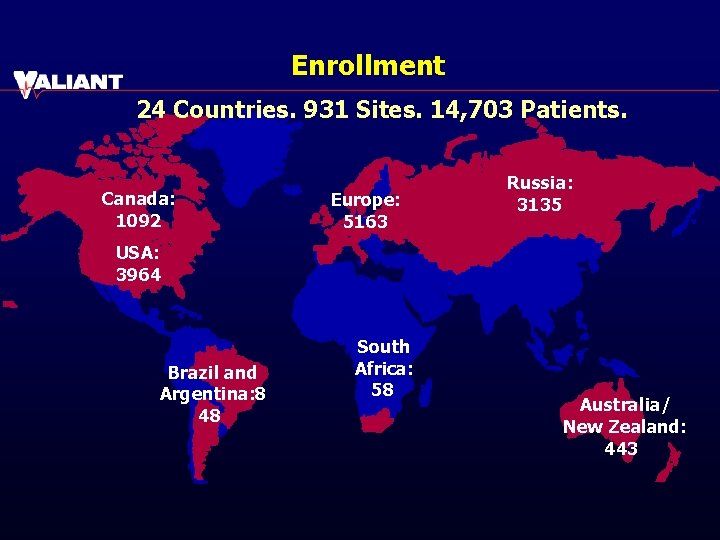
Enrollment 24 Countries. 931 Sites. 14, 703 Patients. Canada: 1092 Europe: 5163 Russia: 3135 USA: 3964 Brazil and Argentina: 8 48 South Africa: 58 Australia/ New Zealand: 443

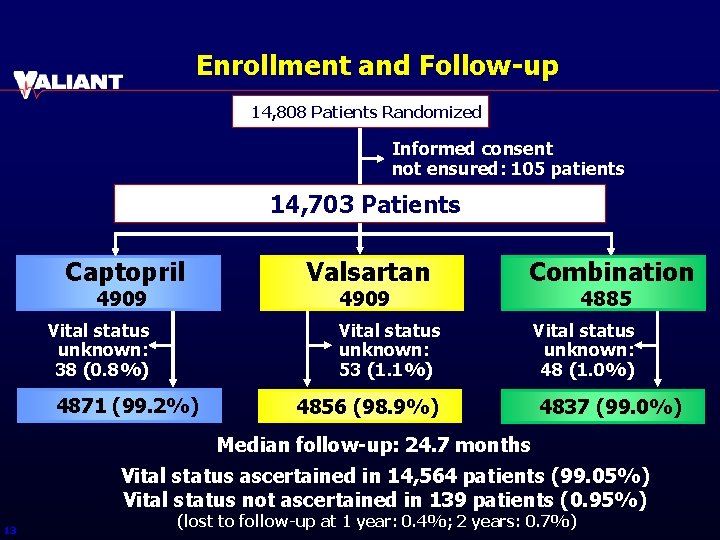
Enrollment and Follow-up 14, 808 Patients Randomized Informed consent not ensured: 105 patients 14, 703 Patients Captopril 4909 Vital status unknown: 38 (0. 8%) Valsartan 4909 Combination Vital status unknown: 53 (1. 1%) 4871 (99. 2%) 4856 (98. 9%) 4885 Vital status unknown: 48 (1. 0%) 4837 (99. 0%) Median follow-up: 24. 7 months Vital status ascertained in 14, 564 patients (99. 05%) Vital status not ascertained in 139 patients (0. 95%) 13 (lost to follow-up at 1 year: 0. 4%; 2 years: 0. 7%)

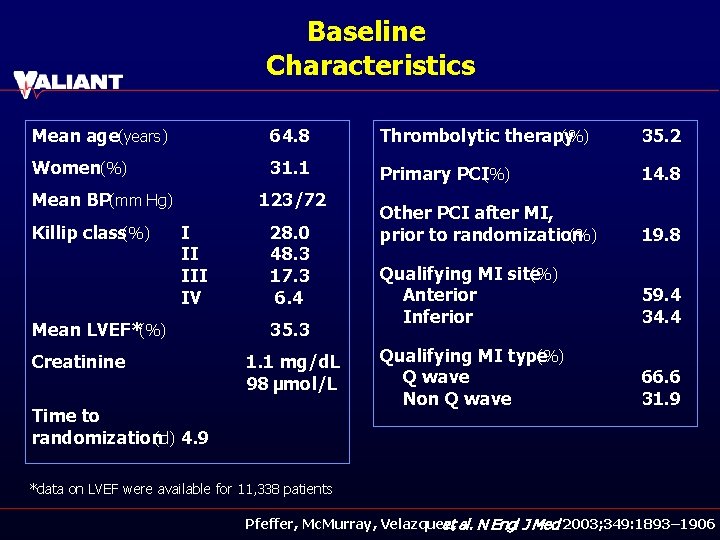
Baseline Characteristics Mean age(years) 64. 8 Thrombolytic therapy (%) 35. 2 Women(%) 31. 1 Primary PCI(%) 14. 8 Other PCI after MI, prior to randomization (%) 19. 8 Qualifying MI site (%) Anterior Inferior 59. 4 34. 4 Qualifying MI type (%) Q wave Non Q wave 66. 6 31. 9 Mean BP(mm Hg) Killip class(%) 123/72 I II IV Mean LVEF*(%) Creatinine 28. 0 48. 3 17. 3 6. 4 35. 3 1. 1 mg/d. L 98 μmol/L Time to randomization(d) 4. 9 *data on LVEF were available for 11, 338 patients Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349: 1893– 1906

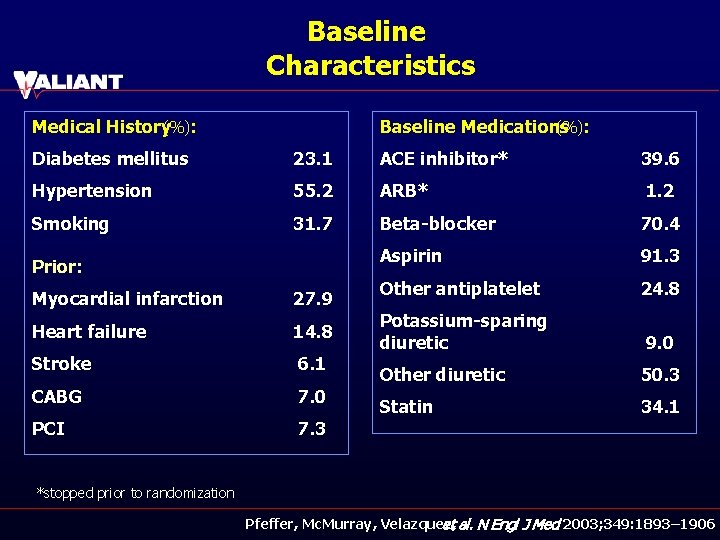
Baseline Characteristics Medical History (%): Baseline Medications (%): Diabetes mellitus 23. 1 ACE inhibitor* 39. 6 Hypertension 55. 2 ARB* 1. 2 Smoking 31. 7 Beta-blocker 70. 4 Aspirin 91. 3 Other antiplatelet 24. 8 Potassium-sparing diuretic 9. 0 Other diuretic 50. 3 Statin 34. 1 Prior: Myocardial infarction 27. 9 Heart failure 14. 8 Stroke 6. 1 CABG 7. 0 PCI 7. 3 *stopped prior to randomization Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349: 1893– 1906

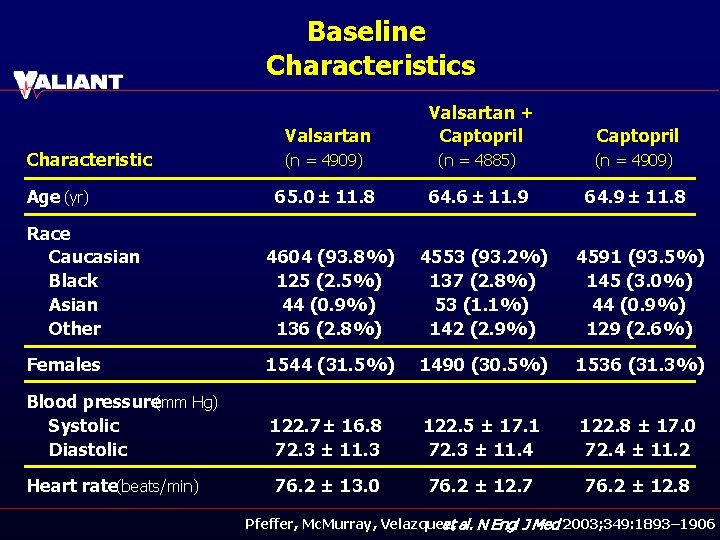
Baseline Characteristics Valsartan Characteristic Valsartan + Captopril (n = 4909) (n = 4885) (n = 4909) 65. 0 ± 11. 8 64. 6 ± 11. 9 64. 9 ± 11. 8 Race Caucasian Black Asian Other 4604 (93. 8%) 125 (2. 5%) 44 (0. 9%) 136 (2. 8%) 4553 (93. 2%) 137 (2. 8%) 53 (1. 1%) 142 (2. 9%) 4591 (93. 5%) 145 (3. 0%) 44 (0. 9%) 129 (2. 6%) Females 1544 (31. 5%) 1490 (30. 5%) 1536 (31. 3%) Blood pressure(mm Hg) Systolic Diastolic 122. 7 ± 16. 8 72. 3 ± 11. 3 122. 5 ± 17. 1 72. 3 ± 11. 4 122. 8 ± 17. 0 72. 4 ± 11. 2 Heart rate(beats/min) 76. 2 ± 13. 0 76. 2 ± 12. 7 76. 2 ± 12. 8 Age (yr) Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349: 1893– 1906

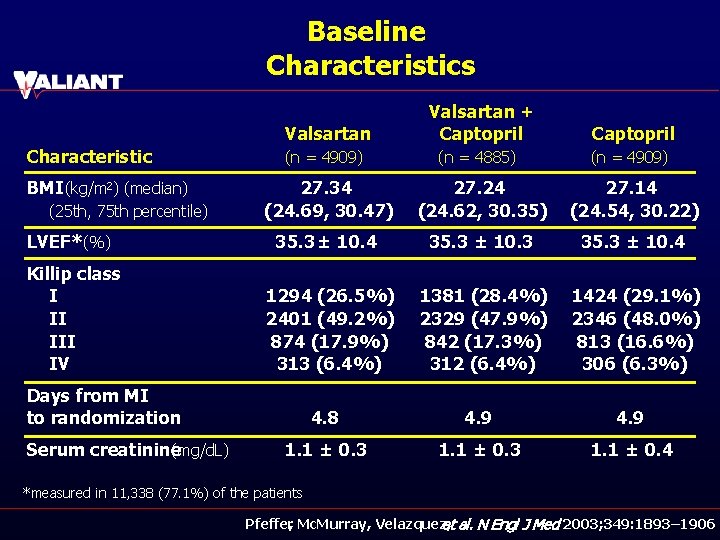
Baseline Characteristics Valsartan Characteristic BMI (kg/m 2) (median) (n = 4909) Valsartan + Captopril (n = 4885) Captopril (n = 4909) 27. 34 (24. 69, 30. 47) 27. 24 (24. 62, 30. 35) 27. 14 (24. 54, 30. 22) LVEF* (%) 35. 3 ± 10. 4 35. 3 ± 10. 3 35. 3 ± 10. 4 Killip class I II IV 1294 (26. 5%) 2401 (49. 2%) 874 (17. 9%) 313 (6. 4%) 1381 (28. 4%) 2329 (47. 9%) 842 (17. 3%) 312 (6. 4%) 1424 (29. 1%) 2346 (48. 0%) 813 (16. 6%) 306 (6. 3%) (25 th, 75 th percentile) Days from MI to randomization Serum creatinine (mg/d. L) 4. 8 4. 9 1. 1 ± 0. 3 1. 1 ± 0. 4 *measured in 11, 338 (77. 1%) of the patients Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349: 1893– 1906

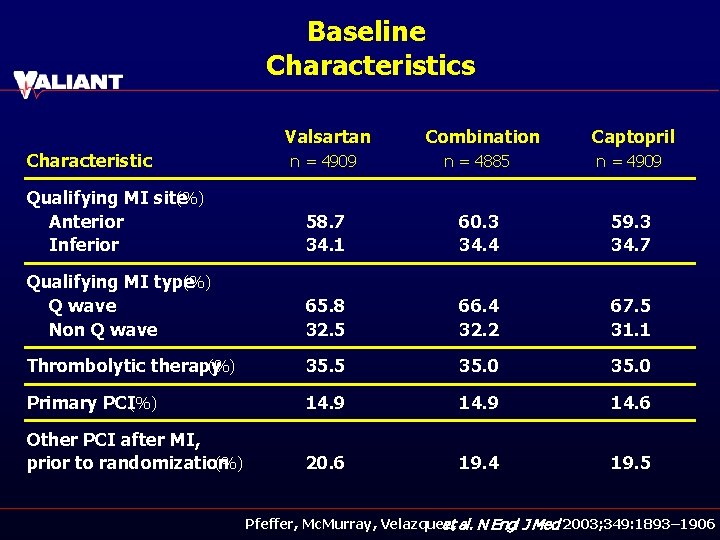
Baseline Characteristics Valsartan Characteristic Combination Captopril n = 4909 n = 4885 n = 4909 Qualifying MI site (%) Anterior Inferior 58. 7 34. 1 60. 3 34. 4 59. 3 34. 7 Qualifying MI type (%) Q wave Non Q wave 65. 8 32. 5 66. 4 32. 2 67. 5 31. 1 Thrombolytic therapy (%) 35. 5 35. 0 Primary PCI(%) 14. 9 14. 6 Other PCI after MI, prior to randomization (%) 20. 6 19. 4 19. 5 Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349: 1893– 1906

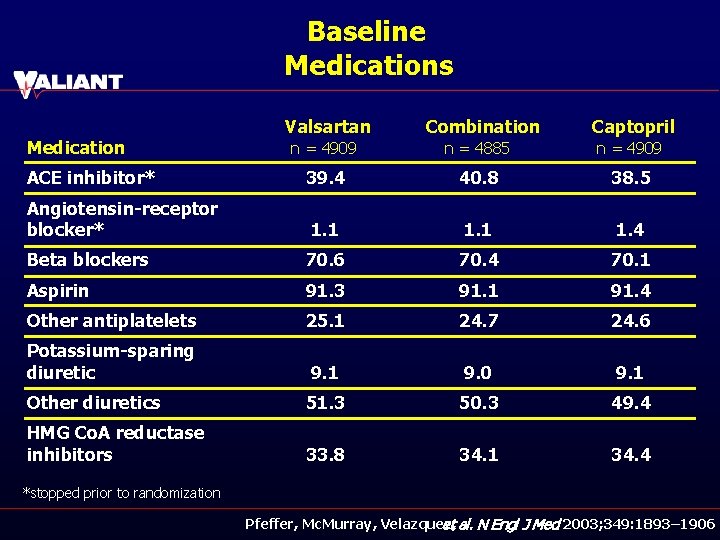
Baseline Medications Medication Valsartan Combination Captopril n = 4909 n = 4885 n = 4909 ACE inhibitor* 39. 4 40. 8 38. 5 Angiotensin-receptor blocker* 1. 1 1. 4 Beta blockers 70. 6 70. 4 70. 1 Aspirin 91. 3 91. 1 91. 4 Other antiplatelets 25. 1 24. 7 24. 6 Potassium-sparing diuretic 9. 1 9. 0 9. 1 Other diuretics 51. 3 50. 3 49. 4 HMG Co. A reductase inhibitors 33. 8 34. 1 34. 4 *stopped prior to randomization Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349: 1893– 1906

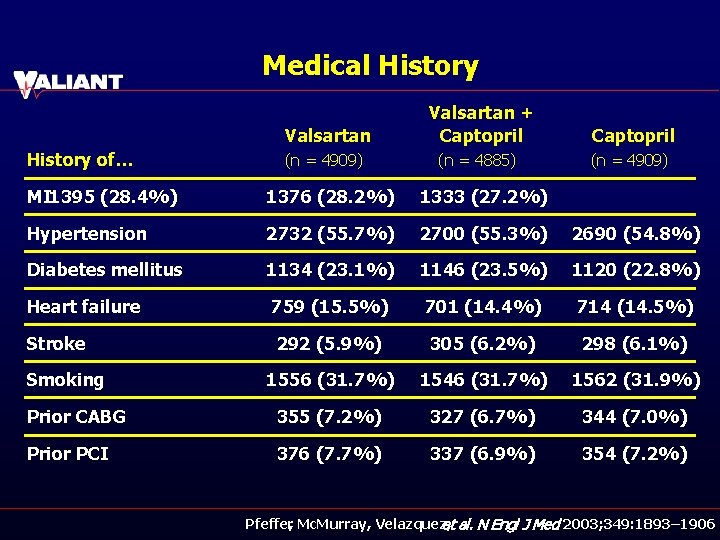
Medical History Valsartan History of… (n = 4909) Valsartan + Captopril (n = 4885) Captopril (n = 4909) MI 1395 (28. 4%) 1376 (28. 2%) 1333 (27. 2%) Hypertension 2732 (55. 7%) 2700 (55. 3%) 2690 (54. 8%) Diabetes mellitus 1134 (23. 1%) 1146 (23. 5%) 1120 (22. 8%) Heart failure 759 (15. 5%) 701 (14. 4%) 714 (14. 5%) Stroke 292 (5. 9%) 305 (6. 2%) 298 (6. 1%) 1556 (31. 7%) 1546 (31. 7%) 1562 (31. 9%) Prior CABG 355 (7. 2%) 327 (6. 7%) 344 (7. 0%) Prior PCI 376 (7. 7%) 337 (6. 9%) 354 (7. 2%) Smoking Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349: 1893– 1906

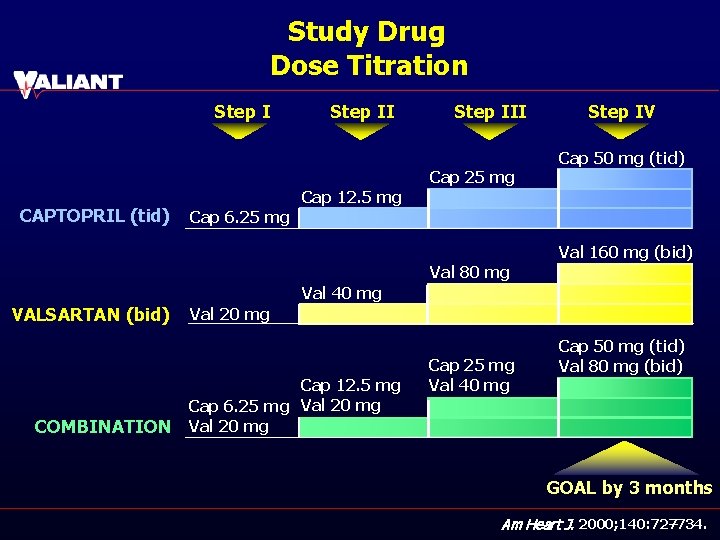
Study Drug Dose Titration Step III Step IV Cap 50 mg (tid) Cap 25 mg CAPTOPRIL (tid) Cap 12. 5 mg Cap 6. 25 mg Val 160 mg (bid) Val 80 mg Val 40 mg VALSARTAN (bid) COMBINATION Val 20 mg Cap 12. 5 mg Cap 6. 25 mg Val 20 mg Cap 25 mg Val 40 mg Cap 50 mg (tid) Val 80 mg (bid) GOAL by 3 months Am Heart J. 2000; 140: 727 – 734.

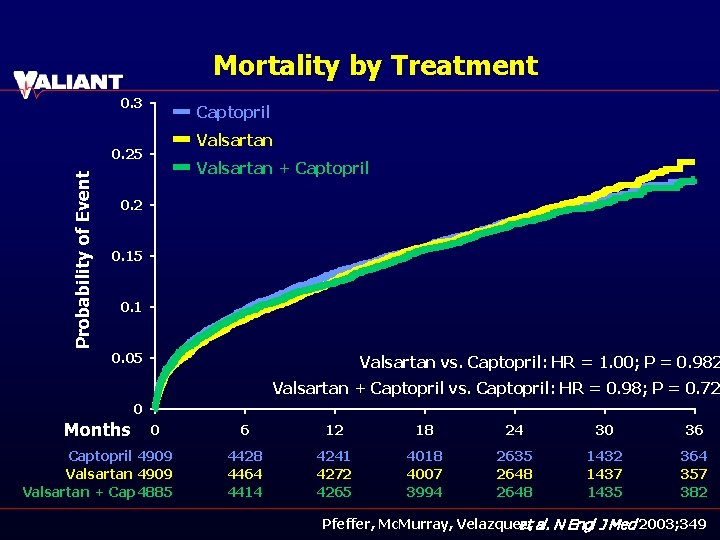
Mortality by Treatment 0. 3 Captopril Valsartan Probability of Event 0. 25 Valsartan + Captopril 0. 2 0. 15 0. 1 0. 05 Valsartan vs. Captopril: HR = 1. 00; P = 0. 982 Valsartan + Captopril vs. Captopril: HR = 0. 98; P = 0. 72 0 Months 0 Captopril 4909 Valsartan + Cap 4885 6 12 18 24 30 36 4428 4464 4414 4241 4272 4265 4018 4007 3994 2635 2648 1432 1437 1435 364 357 382 Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349

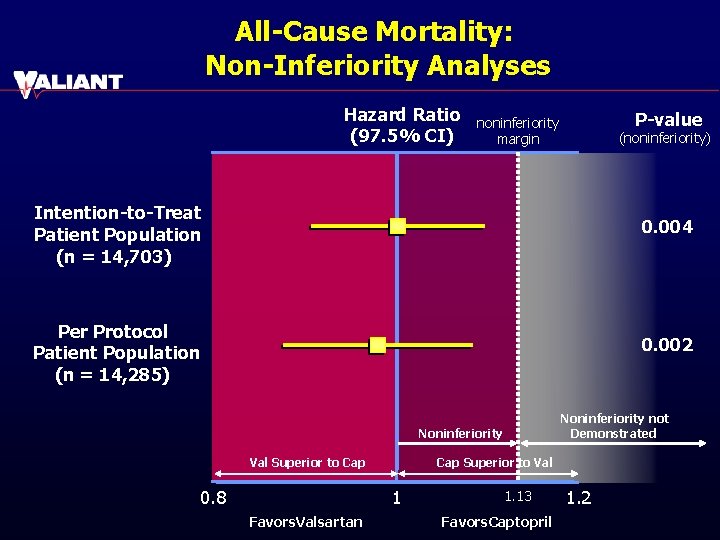
All-Cause Mortality: Non-Inferiority Analyses Hazard Ratio (97. 5% CI) P-value noninferiority margin (noninferiority) Intention-to-Treat Patient Population (n = 14, 703) 0. 004 Per Protocol Patient Population (n = 14, 285) 0. 002 Noninferiority not Demonstrated Noninferiority Val Superior to Cap 0. 8 Cap Superior to Val 1 Favors. Valsartan 1. 13 Favors. Captopril 1. 2

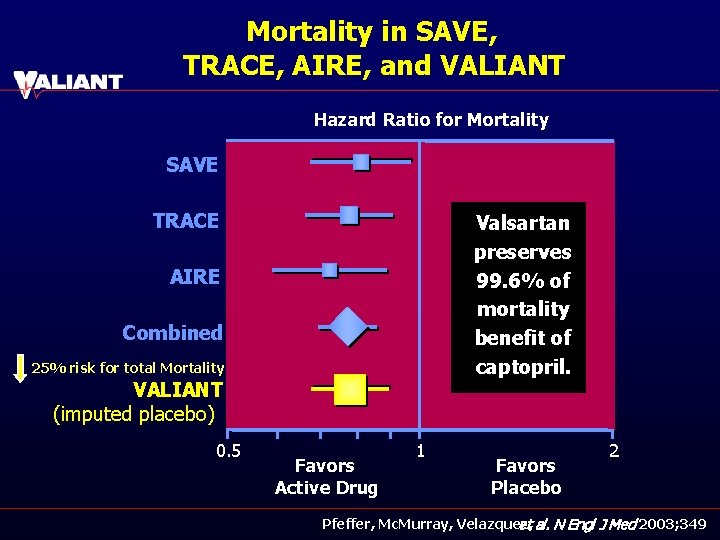
Mortality in SAVE, TRACE, AIRE, and VALIANT Hazard Ratio for Mortality SAVE TRACE Valsartan preserves 99. 6% of mortality benefit of captopril. AIRE Combined 25% risk for total Mortality VALIANT (imputed placebo) 0. 5 Favors Active Drug 1 Favors Placebo 2 Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349

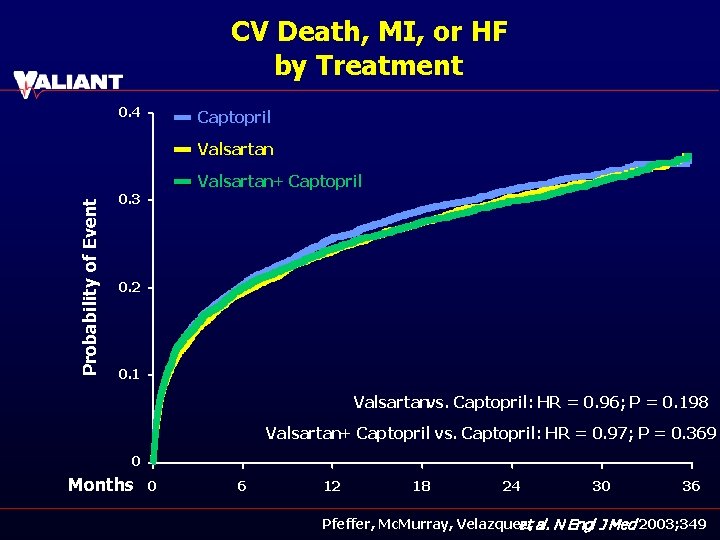
CV Death, MI, or HF by Treatment 0. 4 Captopril Valsartan Probability of Event Valsartan+ Captopril 0. 3 0. 2 0. 1 Valsartanvs. Captopril: HR = 0. 96; P = 0. 198 Valsartan+ Captopril vs. Captopril: HR = 0. 97; P = 0. 369 0 Months 0 6 12 18 24 30 36 Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349

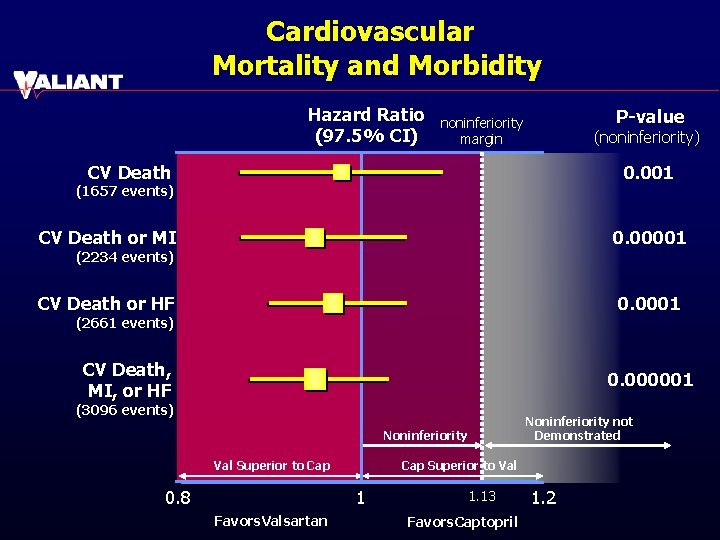
Cardiovascular Mortality and Morbidity Hazard Ratio (97. 5% CI) P-value noninferiority margin (noninferiority) CV Death 0. 001 (1657 events) CV Death or MI 0. 00001 CV Death or HF 0. 0001 (2234 events) (2661 events) CV Death, MI, or HF 0. 000001 (3096 events) Noninferiority not Demonstrated Noninferiority Val Superior to Cap 0. 8 Cap Superior to Val 1 Favors. Valsartan 1. 13 Favors. Captopril 1. 2

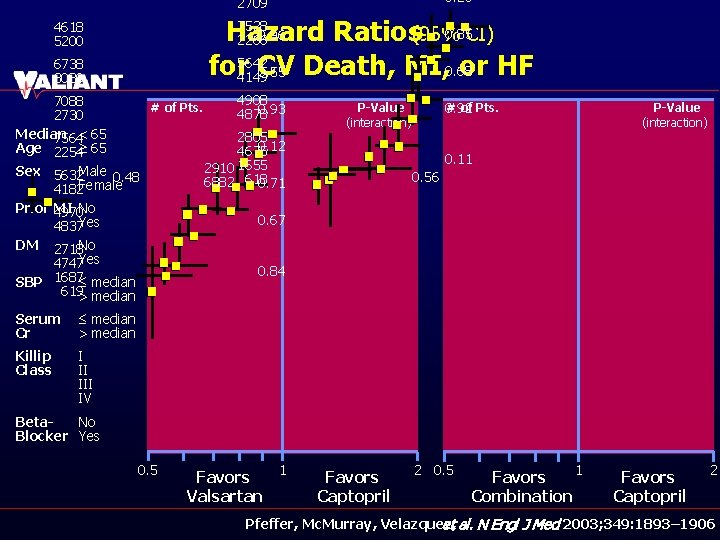
0. 26 2709 7528 0. 85 CI) 0. 96 Hazard Ratios(95% 2266 for 5642 CV or HF 0. 55 Death, MI, 0. 68 4149 4618 5200 6738 3080 4908 7088 # of Pts. 0. 93 4878 2730 Median 2805 7564< 65 0. 12 Age 2254³ 65 4675 2910 1655 Sex 5632 Male 0. 48 6882 618 0. 71 4182 Female Prior 4970 MI No 0. 67 4837 Yes DM 2718 No 4747 Yes 0. 84 SBP 1687£ median 619> median Serum Cr £ median > median Killip Class I II IV # of Pts. 0. 92 P-Value (interaction) 0. 11 0. 56 Beta. No Blocker Yes 0. 5 1 Favors Valsartan Favors Captopril 2 0. 5 1 Favors Combination Favors Captopril 2 Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349: 1893– 1906

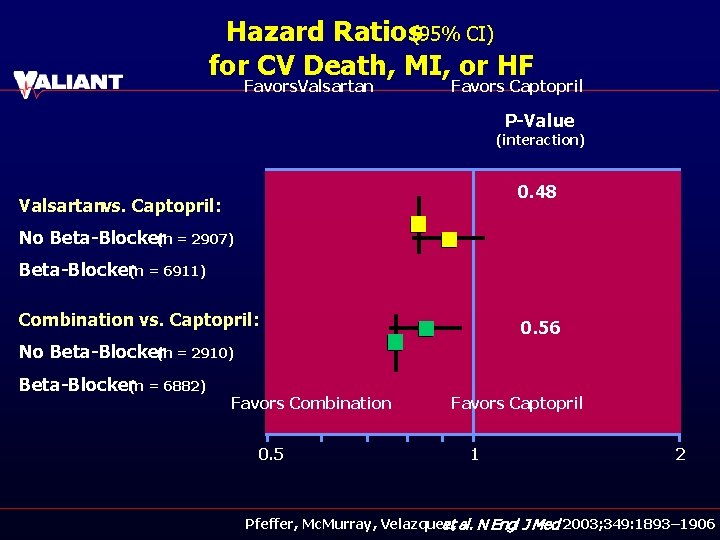
Hazard Ratios(95% CI) for CV Death, MI, or HF Favors. Valsartan Favors Captopril P-Value (interaction) 0. 48 Valsartanvs. Captopril: No Beta-Blocker(n = 2907) Beta-Blocker(n = 6911) Combination vs. Captopril: 0. 56 No Beta-Blocker(n = 2910) Beta-Blocker(n = 6882) Favors Combination 0. 5 Favors Captopril 1 2 Pfeffer, Mc. Murray, Velazquez, et al. N Engl J Med 2003; 349: 1893– 1906

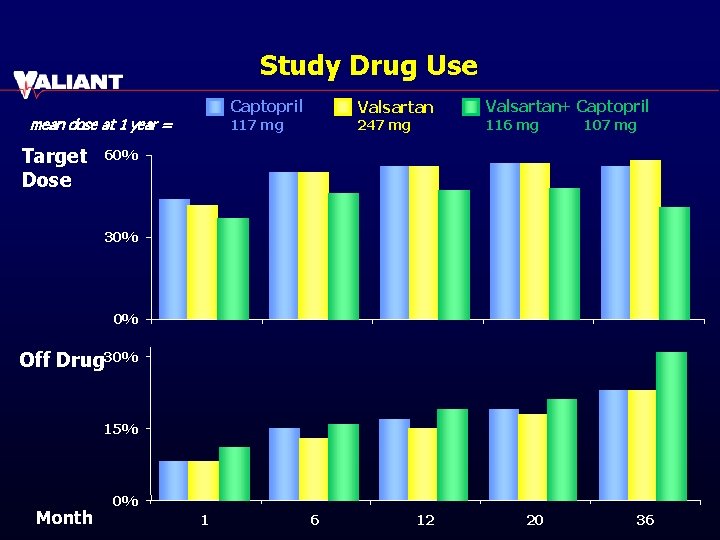
Study Drug Use Captopril mean dose at 1 year = Target Dose Valsartan 117 mg 247 mg Valsartan+ Captopril 116 mg 107 mg 60% 30% 0% Off Drug 30% 15% Month 0% 1 6 12 20 36

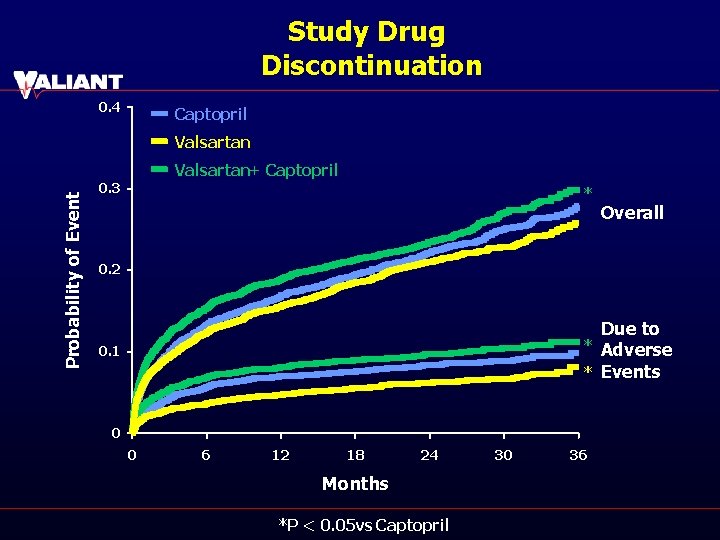
Study Drug Discontinuation 0. 4 Captopril Valsartan Probability of Event Valsartan+ Captopril 0. 3 * Overall 0. 2 * 0. 1 * 0 0 6 12 18 24 Months *P < 0. 05 vs Captopril 30 36 Due to Adverse Events

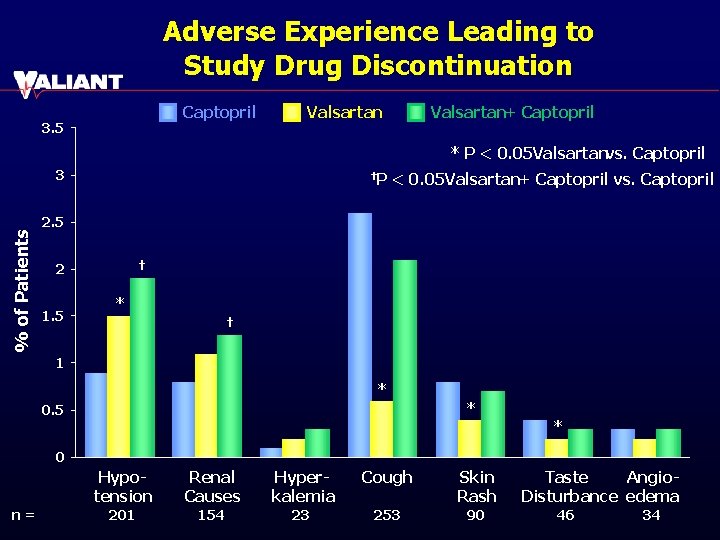
Adverse Experience Leading to Study Drug Discontinuation Captopril 3. 5 Valsartan+ Captopril * P < 0. 05 Valsartanvs. Captopril % of Patients 3 †P < 0. 05 Valsartan+ Captopril vs. Captopril 2. 5 † 2 1. 5 * † 1 * * 0. 5 * 0 n= Hypotension Renal Causes Hyperkalemia Cough Skin Rash 201 154 23 253 90 Taste Angio. Disturbance edema 46 34

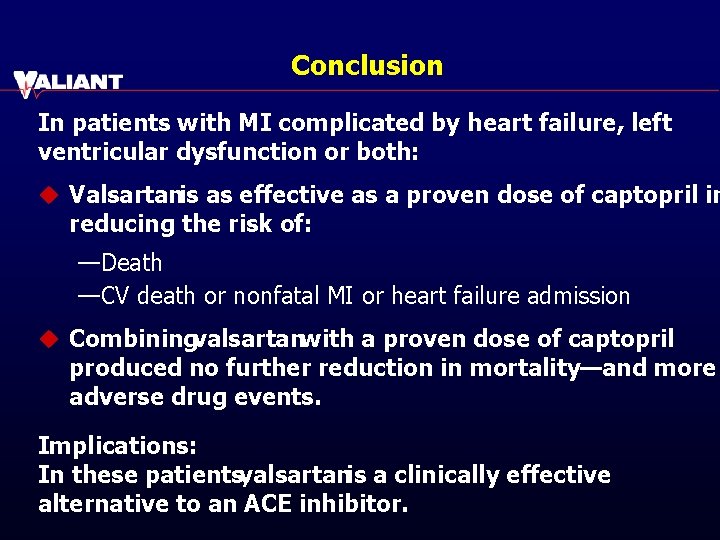
Conclusion In patients with MI complicated by heart failure, left ventricular dysfunction or both: u Valsartanis as effective as a proven dose of captopril in reducing the risk of: —Death —CV death or nonfatal MI or heart failure admission u Combiningvalsartanwith a proven dose of captopril produced no further reduction in mortality—and more adverse drug events. Implications: In these patients, valsartanis a clinically effective alternative to an ACE inhibitor.

Novartis Pharmaceuticals Corporation – Medical Directors : S. Zelenkofske , M. Henis; Project Leader: S. Edwards; Statistician: J. Gong; Programmers : X. Han, J. Shinomoto ; Clinical Team: P. Barbiero, T. Jezek, J. Kaczor, N. B. Keating, R. Koempf, R. Mc. Garry, G. Rossy, C. Salemi, A. Trapani. 24 Countries. 931 Sites. 14, 703 Patients. Thank You
 Valsartan
Valsartan Valsartan + sacubitril
Valsartan + sacubitril Nfarc
Nfarc Hypogastrio
Hypogastrio Myocardial infarction pain location
Myocardial infarction pain location Ecg 1 mm
Ecg 1 mm Tion azi
Tion azi Tion c
Tion c Pancreas wiki
Pancreas wiki Stemi location chart
Stemi location chart Myocardial ischemia meaning
Myocardial ischemia meaning Pico question myocardial infarction
Pico question myocardial infarction Interoperability in the post-acute space
Interoperability in the post-acute space Acute cholecystitis treatment
Acute cholecystitis treatment Periodontitis acuta
Periodontitis acuta Phases of acute glomerulonephritis
Phases of acute glomerulonephritis West haven hepatic encephalopathy
West haven hepatic encephalopathy Acute gingival infections
Acute gingival infections Acute triangle
Acute triangle Acute bronchospasm
Acute bronchospasm Treatments for acute renal failure
Treatments for acute renal failure Acute variables of training
Acute variables of training Joo acute nasopharyngitis
Joo acute nasopharyngitis Adjacent complementary supplementary and vertical angles
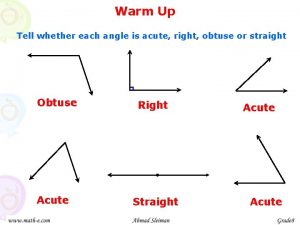
Adjacent complementary supplementary and vertical angles Classify each angle.
Classify each angle. Post traumatic stress disorder
Post traumatic stress disorder Global registry of acute coronary events
Global registry of acute coronary events Acute inflammation
Acute inflammation Acute toxic encephalopathy icd 10
Acute toxic encephalopathy icd 10 Difference between acute and subacute rehab
Difference between acute and subacute rehab Acute mylogenous leukemia
Acute mylogenous leukemia