Vaccine Services Update Karen Hess Vaccine Services Manager















- Slides: 32

Vaccine Services Update Karen Hess Vaccine Services Manager February 19, 2009

Topics n. Providers n. NIS n. Vaccine Update n. Electronic Vaccine Accounting n. Non-Compliance Policy


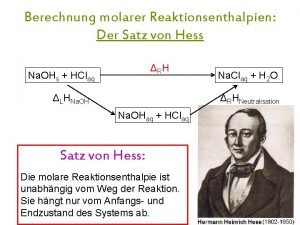
2007 Co-CASA Rates

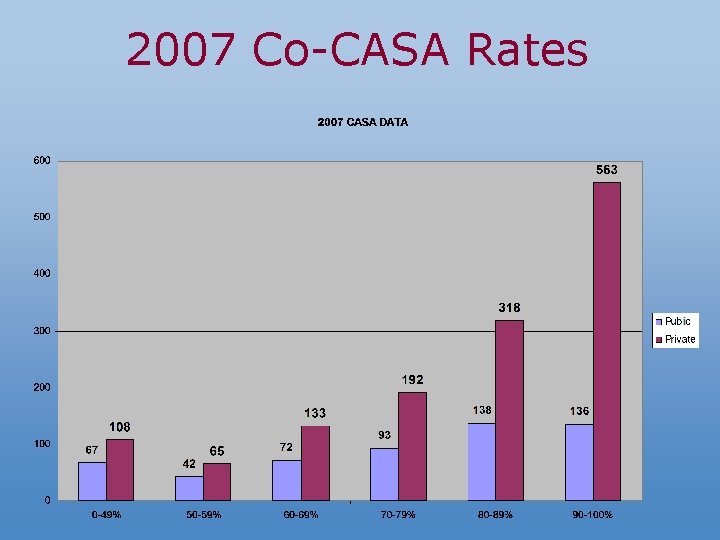
Public vs. Private Recruitment Doses Administered Percentage

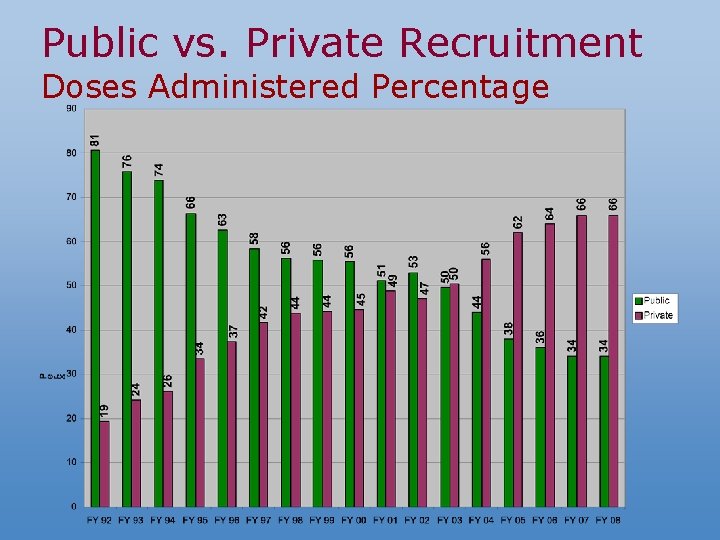
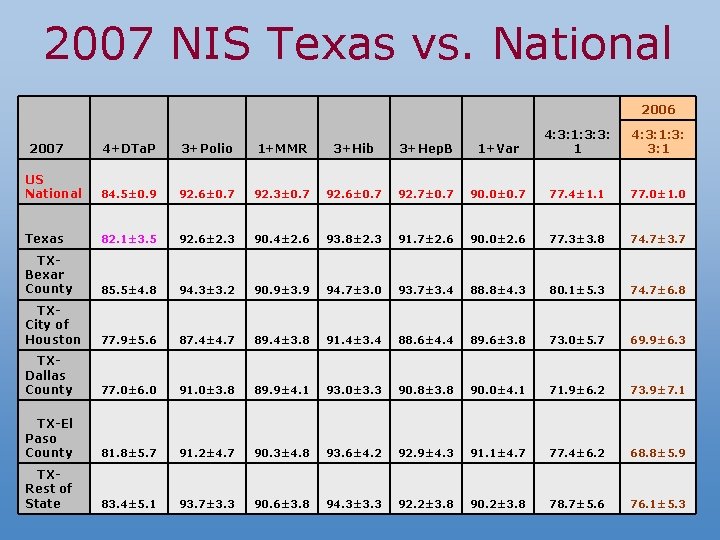
2007 NIS Texas vs. National 2006 4+DTa. P 3+Polio 1+MMR 3+Hib 3+Hep. B 1+Var 4: 3: 1: 3: 3: 1 US National 84. 5± 0. 9 92. 6± 0. 7 92. 3± 0. 7 92. 6± 0. 7 92. 7± 0. 7 90. 0± 0. 7 77. 4± 1. 1 77. 0± 1. 0 Texas 82. 1± 3. 5 92. 6± 2. 3 90. 4± 2. 6 93. 8± 2. 3 91. 7± 2. 6 90. 0± 2. 6 77. 3± 3. 8 74. 7± 3. 7 TXBexar County 85. 5± 4. 8 94. 3± 3. 2 90. 9± 3. 9 94. 7± 3. 0 93. 7± 3. 4 88. 8± 4. 3 80. 1± 5. 3 74. 7± 6. 8 TXCity of Houston 77. 9± 5. 6 87. 4± 4. 7 89. 4± 3. 8 91. 4± 3. 4 88. 6± 4. 4 89. 6± 3. 8 73. 0± 5. 7 69. 9± 6. 3 TXDallas County 77. 0± 6. 0 91. 0± 3. 8 89. 9± 4. 1 93. 0± 3. 3 90. 8± 3. 8 90. 0± 4. 1 71. 9± 6. 2 73. 9± 7. 1 TX-El Paso County 81. 8± 5. 7 91. 2± 4. 7 90. 3± 4. 8 93. 6± 4. 2 92. 9± 4. 3 91. 1± 4. 7 77. 4± 6. 2 68. 8± 5. 9 TXRest of State 83. 4± 5. 1 93. 7± 3. 3 90. 6± 3. 8 94. 3± 3. 3 92. 2± 3. 8 90. 2± 3. 8 78. 7± 5. 6 76. 1± 5. 3 2007 4: 3: 1: 3: 3: 1

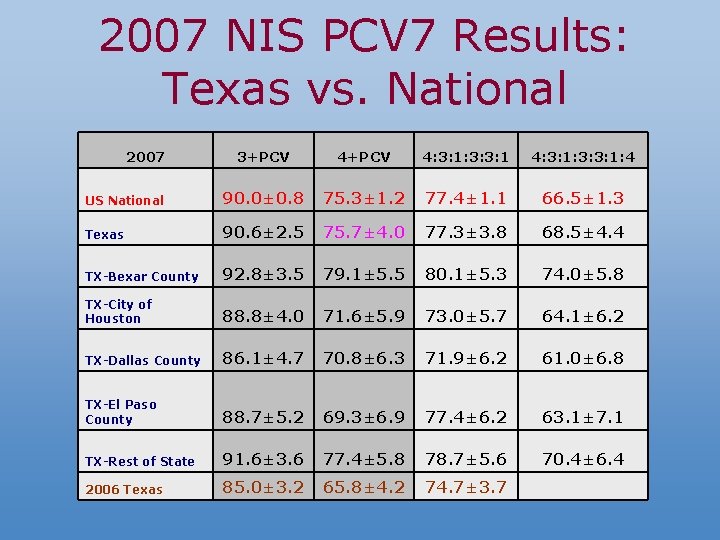
2007 NIS PCV 7 Results: Texas vs. National 2007 3+PCV 4: 3: 1: 3: 3: 1: 4 US National 90. 0± 0. 8 75. 3± 1. 2 77. 4± 1. 1 66. 5± 1. 3 Texas 90. 6± 2. 5 75. 7± 4. 0 77. 3± 3. 8 68. 5± 4. 4 TX-Bexar County 92. 8± 3. 5 79. 1± 5. 5 80. 1± 5. 3 74. 0± 5. 8 TX-City of Houston 88. 8± 4. 0 71. 6± 5. 9 73. 0± 5. 7 64. 1± 6. 2 TX-Dallas County 86. 1± 4. 7 70. 8± 6. 3 71. 9± 6. 2 61. 0± 6. 8 TX-El Paso County 88. 7± 5. 2 69. 3± 6. 9 77. 4± 6. 2 63. 1± 7. 1 TX-Rest of State 91. 6± 3. 6 77. 4± 5. 8 78. 7± 5. 6 70. 4± 6. 4 2006 Texas 85. 0± 3. 2 65. 8± 4. 2 74. 7± 3. 7

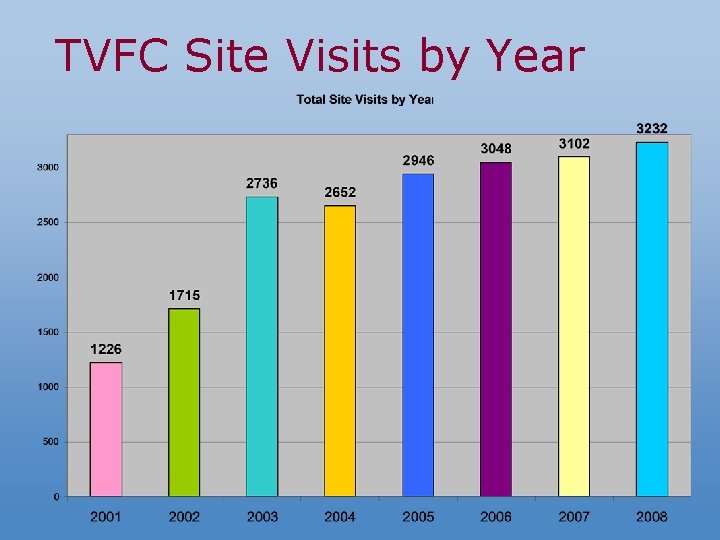
TVFC Site Visits by Year

Vaccines Shipped by Year

Vaccines Shipped 2006 -2008


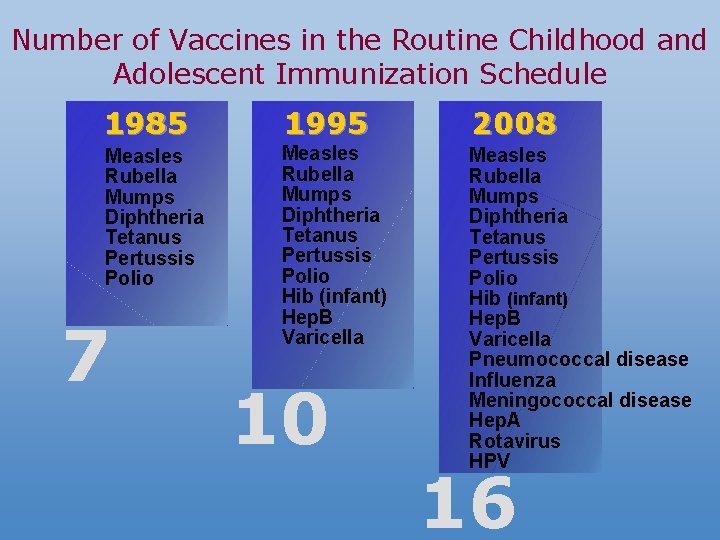
Number of Vaccines in the Routine Childhood and Adolescent Immunization Schedule 1985 1995 2008 Measles Rubella Mumps Diphtheria Tetanus Pertussis Polio Hib (infant) Hep. B Varicella Pneumococcal disease Influenza Meningococcal disease Hep. A Rotavirus HPV 7 10 16

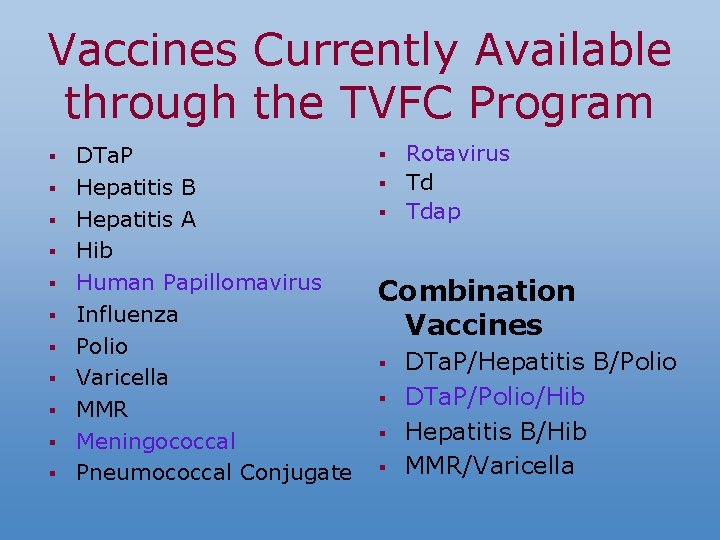
Vaccines Currently Available through the TVFC Program § § § DTa. P Hepatitis B Hepatitis A Hib Human Papillomavirus Influenza Polio Varicella MMR Meningococcal Pneumococcal Conjugate § § § Rotavirus Td Tdap Combination Vaccines § § DTa. P/Hepatitis B/Polio DTa. P/Polio/Hib Hepatitis B/Hib MMR/Varicella

Last Three Years In Vaccine Releases n 3 Newly Licensed Vaccines in 2006 n n n Gardasil®, Zostavax®, Rota. Teq® 2 Newly Licensed Vaccines in 2007 n Menactra™ (2 -10 years) n Flu. Mist® (5 -49 years) 4 Newly Licensed Vaccines in 2008 n Kinrix®, Pentacel®, Rotarix®, BOOSTRIX® (for 19 -65 year olds), Flu. Mist® (2 -49 years) As of 1/20/2009

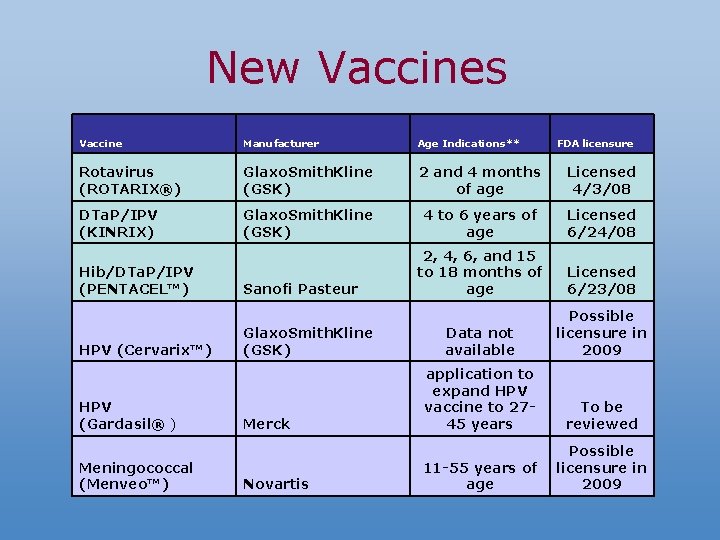
New Vaccines Vaccine Manufacturer Age Indications** Rotavirus (ROTARIX®) Glaxo. Smith. Kline (GSK) 2 and 4 months of age Licensed 4/3/08 DTa. P/IPV (KINRIX) Glaxo. Smith. Kline (GSK) 4 to 6 years of age Licensed 6/24/08 2, 4, 6, and 15 to 18 months of age Licensed 6/23/08 Data not available Possible licensure in 2009 application to expand HPV vaccine to 2745 years To be reviewed 11 -55 years of age Possible licensure in 2009 Hib/DTa. P/IPV (PENTACEL™) HPV (Cervarix™) HPV (Gardasil® ) Meningococcal (Menveo™) Sanofi Pasteur Glaxo. Smith. Kline (GSK) Merck Novartis FDA licensure

Pedvax. Hib n. Improvement ® Shortage in the supply is not expected until summer 2009 n. The booster dose of Hib vaccine usually administered at 12 -15 months of age should be deferred except for children at increased risk of Hib disease n asplenia n sickle cell disease n immunodeficiency (including HIV infection and cancer) n American Indian children n Alaska Native children Note: DSHS distributes the 3 dose Act. Hib ® product only.

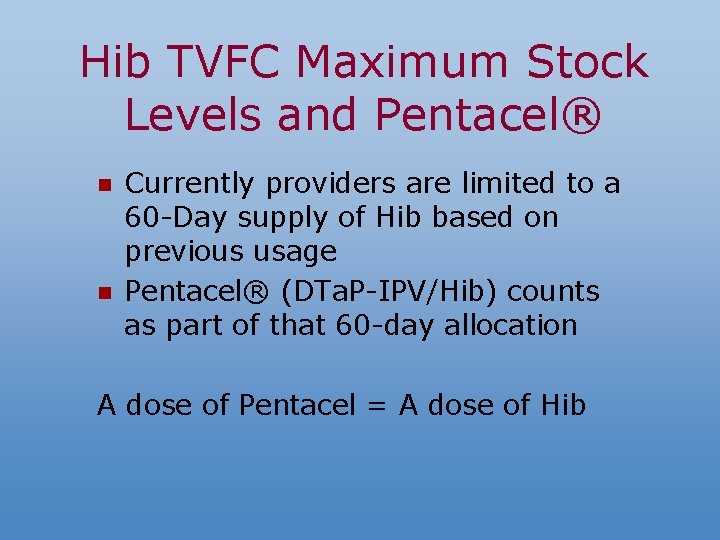
Hib TVFC Maximum Stock Levels and Pentacel® n n Currently providers are limited to a 60 -Day supply of Hib based on previous usage Pentacel® (DTa. P-IPV/Hib) counts as part of that 60 -day allocation A dose of Pentacel = A dose of Hib

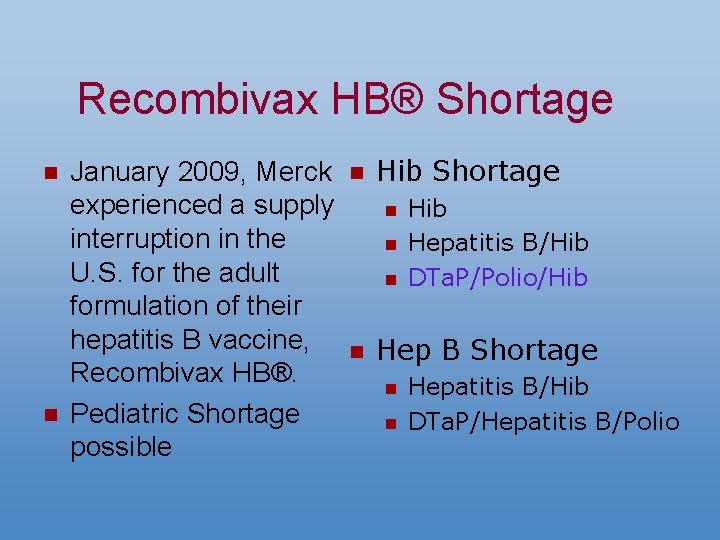
Recombivax HB® Shortage n n January 2009, Merck experienced a supply interruption in the U. S. for the adult formulation of their hepatitis B vaccine, Recombivax HB®. Pediatric Shortage possible n Hib Shortage n n Hib Hepatitis B/Hib DTa. P/Polio/Hib Hep B Shortage n n Hepatitis B/Hib DTa. P/Hepatitis B/Polio

Electronic Vaccine Accounting (EVA) n CDC New Vaccine Ordering System n n n Vaccine Choice Legislation n New reporting elements On-line ordering and reporting for all TVFC providers. Increase Brand Options Add presentation (syringes, vials) Simplify ordering n n Create “recommended order” Auto mathematical calculation features Pre-population of previously entered inventory information (lot number, NDC, expiration date) Automated reminder

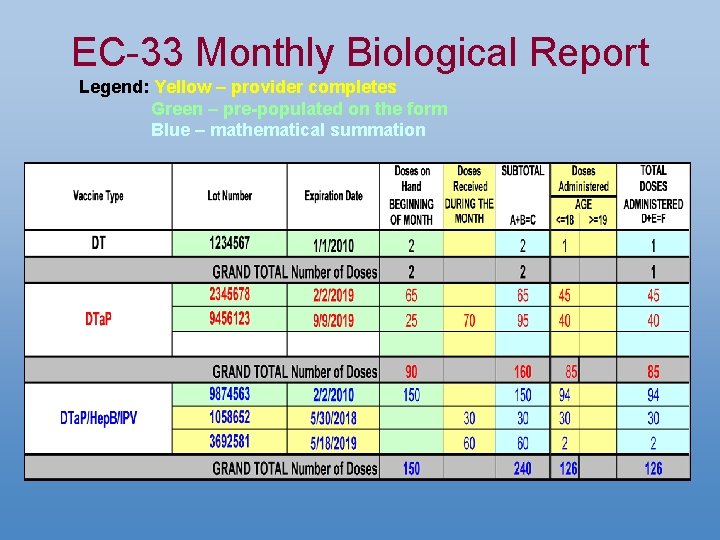
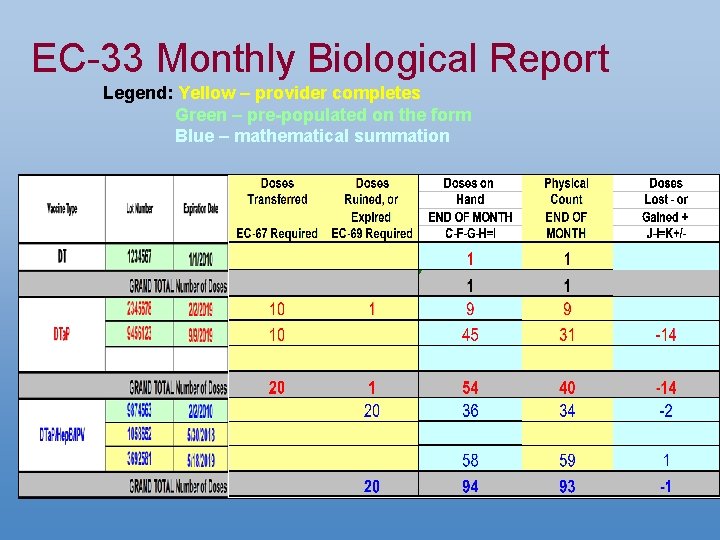
EC-33 Monthly Biological Report Legend: Yellow – provider completes Green – pre-populated on the form Blue – mathematical summation

EC-33 Monthly Biological Report Legend: Yellow – provider completes Green – pre-populated on the form Blue – mathematical summation

Biological Order Form

EVA - Next Steps Request capital authority n Contract out for bid n Workgroup formed n Focus group and pilot testing n Goal by September 2010 n

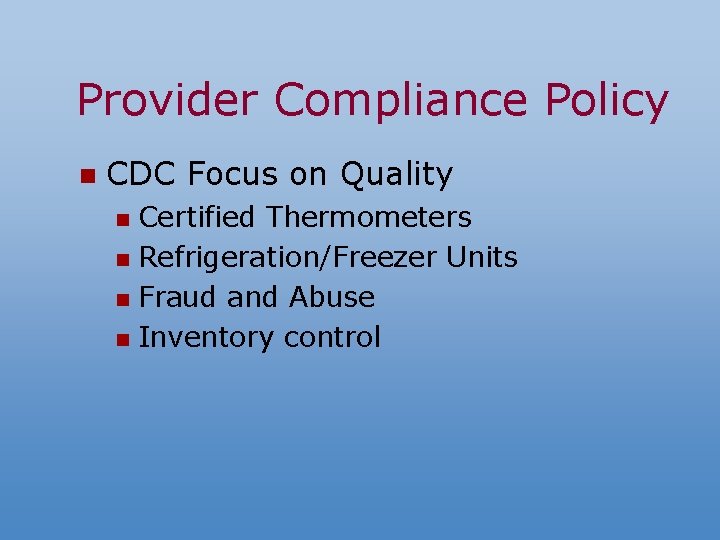
Provider Compliance Policy n CDC Focus on Quality Certified Thermometers n Refrigeration/Freezer Units n Fraud and Abuse n Inventory control n

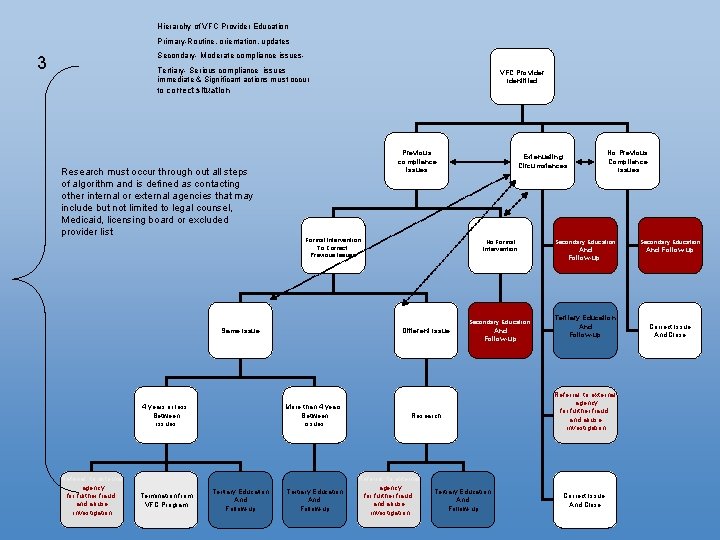
Hierarchy of VFC Provider Education Primary-Routine, orientation, updates Secondary- Moderate compliance issues- 3 Tertiary- Serious compliance issues immediate & Significant actions must occur to correct situation Research must occur through out all steps of algorithm and is defined as contacting other internal or external agencies that may include but not limited to legal counsel, Medicaid, licensing board or excluded provider list VFC Provider Identified Previous compliance issues Formal Intervention To Correct Previous Issues Extenuating Circumstances No Formal Intervention Secondary Education Same Issue More than 4 years Between issues 4 years or less Between issues Referral to external agency for further fraud and abuse investigation Termination from VFC Program Different Issue Tertiary Education And Follow-up Research Referral to external agency for further fraud and abuse investigation Tertiary Education And Follow-up No Previous Compliance Issues Secondary Education And Follow-up Tertiary Education And Follow-up Referral to external agency for further fraud and abuse investigation Correct Issue And Close

Provider Compliance Policy Purpose n Consistent process when handling TVFC compliance issues n Identify high risk non-compliance issues n Protect TVFC from potential fraud and abuse n Prevents re-occurrence with sufficient training, education, and documentation n Identifies need to refer providers to the Texas State Office of Inspector General (OIG), or terminate from the TVFC program

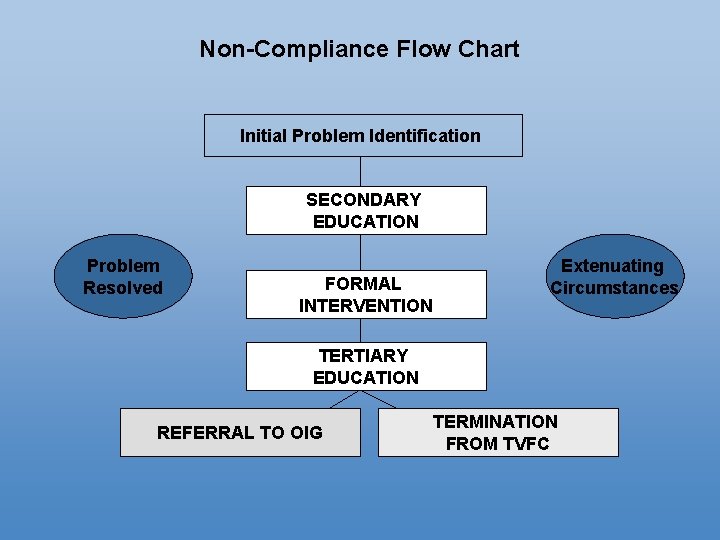
Non-Compliance Flow Chart Initial Problem Identification SECONDARY EDUCATION Problem Resolved FORMAL INTERVENTION Extenuating Circumstances TERTIARY EDUCATION REFERRAL TO OIG TERMINATION FROM TVFC

Provider Compliance Policy Format n Policy n Definitions n Procedures Non-Compliance Identified During a On. Site Visit n Non-Compliance Identified by Other Means n

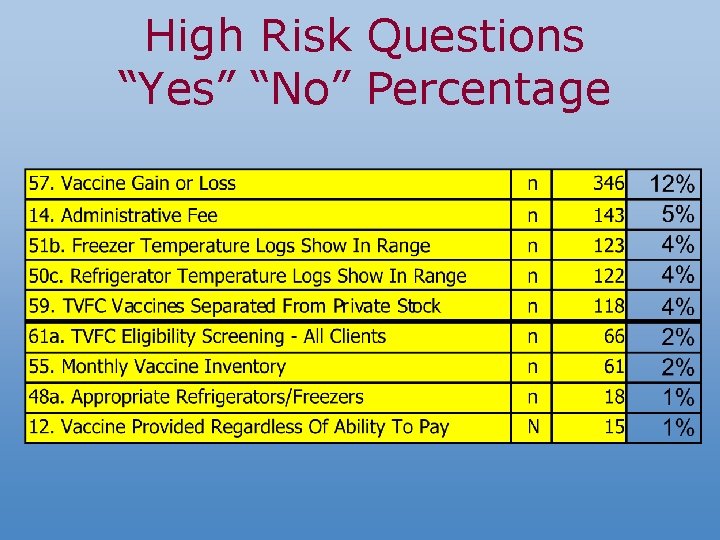
10 High Risk Questions n n n n n 12. Vaccines provided regardless of inability to pay 14. Administration fee is less than or equal to the maximum fee 48 a. Appropriate refrigerators/freezers are used to store vaccine 50 a. Refrigerator/freezer temperature log is available for the last three months 50 d. From the logs provided, all recorded refrigerator temperatures are within the recommended range. 51 c. From the logs provided, all recorded freezer temperatures are within the recommended range. 55. A physical inventory of vaccine is done monthly 57. The loss/gain is less than 5% 59. TVFC vaccines can be distinguished from private stock 61 a. All clients are screened for eligibility

High Risk Questions “Yes” “No” Percentage

Compliance Policy Feedback Operations Manual Appendix n Gauge Success n CDC and HSR/LHD feedback n

Thank You
 Shadow paging recovery technique
Shadow paging recovery technique Airport manager karen
Airport manager karen Senior manager vs general manager
Senior manager vs general manager Portfolio manager synergy manager parental developer
Portfolio manager synergy manager parental developer Q mc ∆ t
Q mc ∆ t Application of hess law
Application of hess law Ib chemistry topic 5
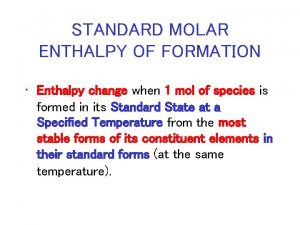
Ib chemistry topic 5 Enthalpy change unit
Enthalpy change unit Sea floor spreading drawing
Sea floor spreading drawing Arthur holmes contribution to plate tectonics
Arthur holmes contribution to plate tectonics Module 3 lesson 6
Module 3 lesson 6 Aspirateur daniel hess
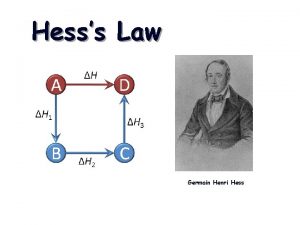
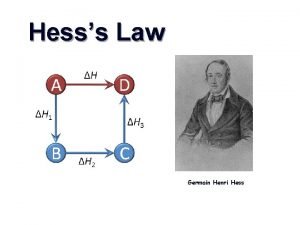
Aspirateur daniel hess Hess law
Hess law Hess's law example
Hess's law example Hess cycle questions
Hess cycle questions Golftini owner
Golftini owner Enthalpy change of reaction
Enthalpy change of reaction Senior high school subjects (per semester)
Senior high school subjects (per semester) Harry hess and seafloor spreading
Harry hess and seafloor spreading Satz von hess
Satz von hess Hunt and hess scoring
Hunt and hess scoring Hess's law rules
Hess's law rules Fischl amy
Fischl amy Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Trabajo mecanico
Trabajo mecanico David c. hess, md
David c. hess, md Hess expanded on wegener’s theory of .
Hess expanded on wegener’s theory of . Hess
Hess Hess law definition
Hess law definition Hess law constant heat summation
Hess law constant heat summation Hess law definition
Hess law definition Hess law constant heat summation
Hess law constant heat summation Hess law constant heat summation
Hess law constant heat summation