Agenda 228 1 Hess law notes 2 Hess

- Slides: 7

Agenda 2/28 1. Hess’ law notes 2. Hess’ Law worksheet 3. Finish lab Homework: Lab report, test Friday, Hess’ law worksheet

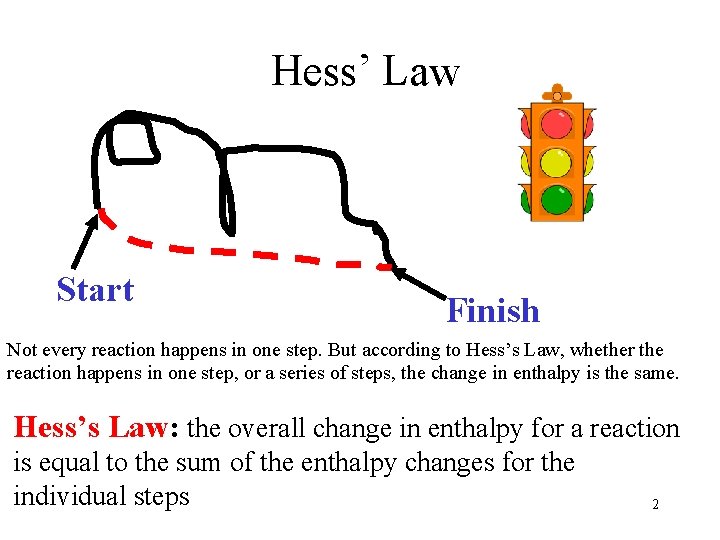
Hess’ Law Start Finish Not every reaction happens in one step. But according to Hess’s Law, whether the reaction happens in one step, or a series of steps, the change in enthalpy is the same. Hess’s Law: the overall change in enthalpy for a reaction is equal to the sum of the enthalpy changes for the individual steps 2

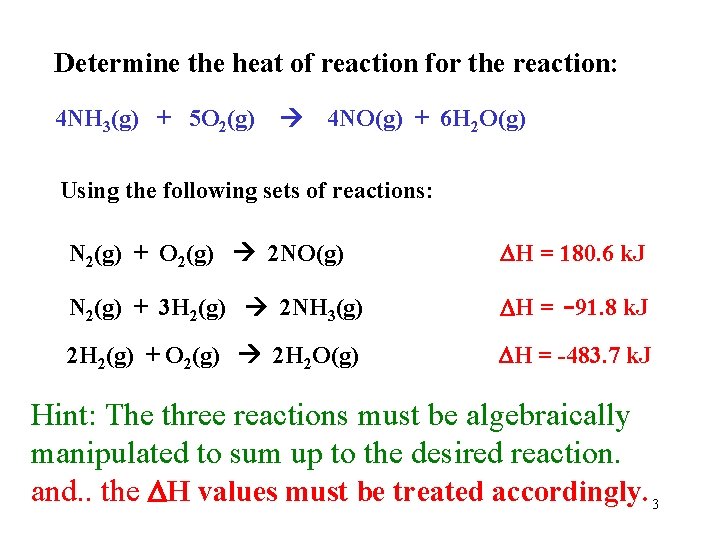
Determine the heat of reaction for the reaction: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) Using the following sets of reactions: N 2(g) + O 2(g) 2 NO(g) H = 180. 6 k. J N 2(g) + 3 H 2(g) 2 NH 3(g) H = -91. 8 k. J 2 H 2(g) + O 2(g) 2 H 2 O(g) H = -483. 7 k. J Hint: The three reactions must be algebraically manipulated to sum up to the desired reaction. and. . the H values must be treated accordingly. 3

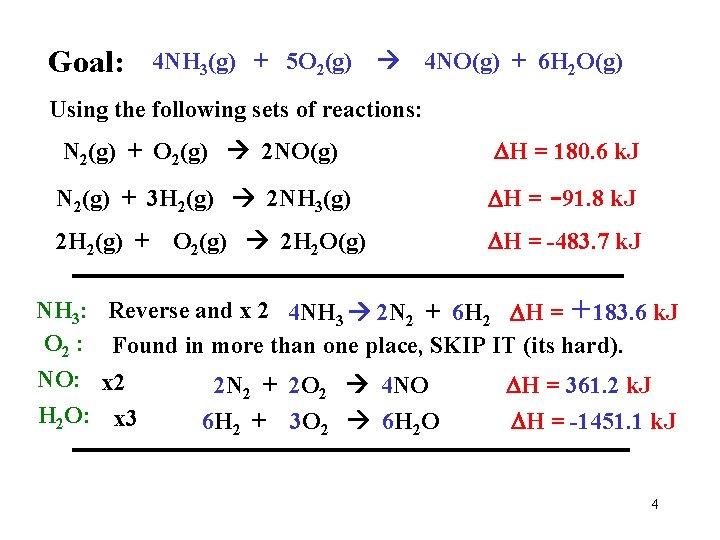
Goal: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) Using the following sets of reactions: N 2(g) + O 2(g) 2 NO(g) H = 180. 6 k. J N 2(g) + 3 H 2(g) 2 NH 3(g) H = -91. 8 k. J 2 H 2(g) + NH 3: O 2 : NO: H 2 O: O 2(g) 2 H 2 O(g) H = -483. 7 k. J Reverse and x 2 4 NH 3 2 N 2 + 6 H 2 H = +183. 6 k. J Found in more than one place, SKIP IT (its hard). x 2 x 3 2 N 2 + 2 O 2 4 NO 6 H 2 + 3 O 2 6 H 2 O H = 361. 2 k. J H = -1451. 1 k. J 4

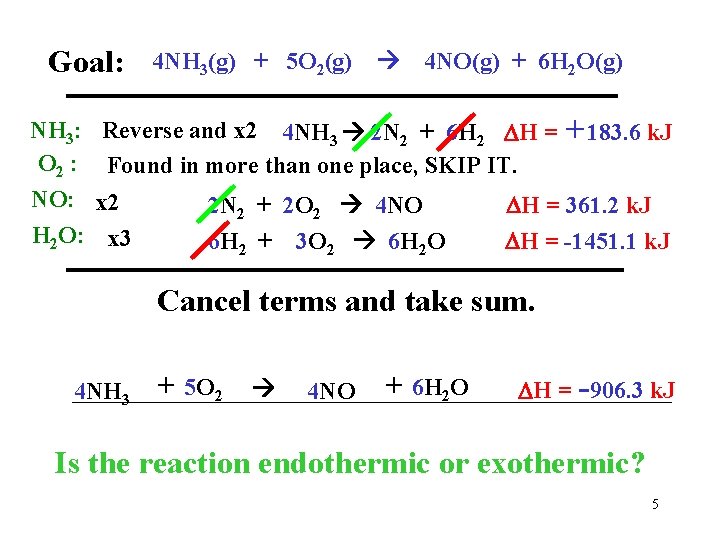
Goal: NH 3: O 2 : NO: H 2 O: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) Reverse and x 2 4 NH 3 2 N 2 + 6 H 2 H = Found in more than one place, SKIP IT. x 2 x 3 2 N 2 + 2 O 2 4 NO 6 H 2 + 3 O 2 6 H 2 O +183. 6 k. J H = 361. 2 k. J H = -1451. 1 k. J Cancel terms and take sum. 4 NH 3 + 5 O 2 4 NO + 6 H 2 O H = -906. 3 k. J Is the reaction endothermic or exothermic? 5

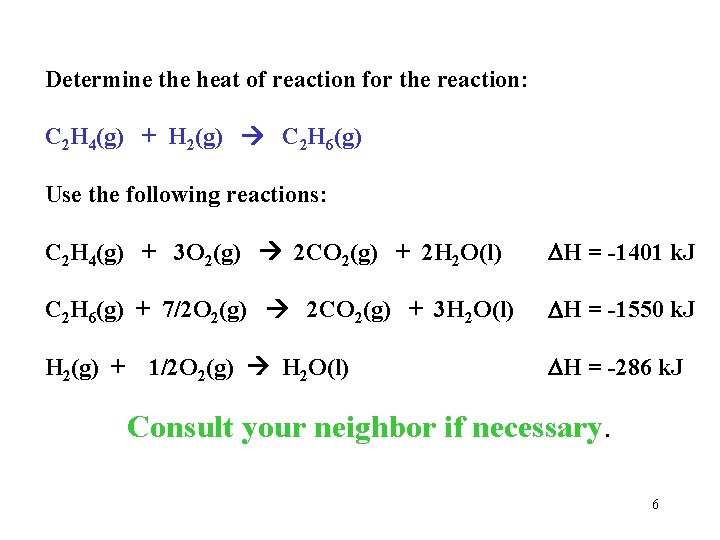
Determine the heat of reaction for the reaction: C 2 H 4(g) + H 2(g) C 2 H 6(g) Use the following reactions: C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) H = -1401 k. J C 2 H 6(g) + 7/2 O 2(g) 2 CO 2(g) + 3 H 2 O(l) H = -1550 k. J H 2(g) + 1/2 O 2(g) H 2 O(l) H = -286 k. J Consult your neighbor if necessary. 6

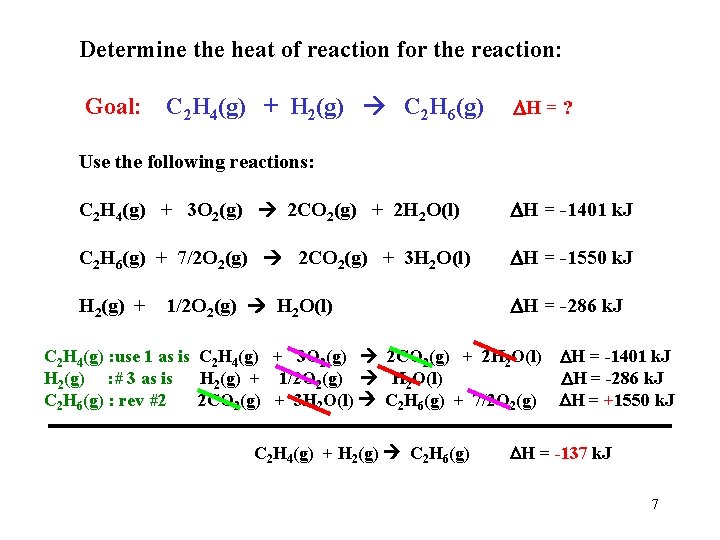
Determine the heat of reaction for the reaction: Goal: C 2 H 4(g) + H 2(g) C 2 H 6(g) H = ? Use the following reactions: C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) H = -1401 k. J C 2 H 6(g) + 7/2 O 2(g) 2 CO 2(g) + 3 H 2 O(l) H = -1550 k. J H 2(g) + 1/2 O 2(g) H 2 O(l) H = -286 k. J C 2 H 4(g) : use 1 as is C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) H = -1401 k. J H 2(g) : # 3 as is H 2(g) + 1/2 O 2(g) H 2 O(l) H = -286 k. J C 2 H 6(g) : rev #2 2 CO 2(g) + 3 H 2 O(l) C 2 H 6(g) + 7/2 O 2(g) H = +1550 k. J C 2 H 4(g) + H 2(g) C 2 H 6(g) H = -137 k. J 7