Vaccination against pertussis whooping cough for pregnant women




































- Slides: 44

Vaccination against pertussis (whooping cough) for pregnant women An update for healthcare professionals May 2014

Key Message • Infant cases of pertussis have been dramatically reduced by the introduction of the temporary maternal immunisation programme. However, the disease continues to circulate in older age groups • Therefore it is important for all health professionals to encourage vaccination in pregnant women from 28 weeks, at the earliest opportunity © NHS Scotland/Crown Copyright 2 Vaccination against pertussis for pregnant women

Aims of Resource • To support staff involved in discussing vaccination against pertussis with pregnant women by providing evidence based information • To raise awareness of current pertussis epidemiology and the impact of pertussis on young infants • To promote uptake of vaccination against pertussis through increasing awareness amongst healthcare professionals © NHS Scotland/Crown Copyright 3 Vaccination against pertussis for pregnant women 3

Learning Outcomes After completing this resource immunisers will be able to: • Understand their role in raising the issue of vaccination against pertussis with all women in the antenatal period and providing women with evidence based information about this vaccination • Describe the aetiology and epidemiology of pertussis • Understand how pertussis is transmitted and the severity of it in young infants • Discuss the important role of vaccination against pertussis during pregnancy for young infants • Be aware of sources of additional information 4 Vaccination against pertussis for pregnant women

Contents 1. What is pertussis? 2. Why vaccinate pregnant women against pertussis? 3. Vaccination against pertussis and the use of Boostrix® 4. The role of the immuniser 5. Resources 5 Vaccination against pertussis for pregnant women

What is pertussis? 6 Vaccination against pertussis for pregnant women

What is pertussis? • Pertussis is an acute bacterial infection caused by Bordetella pertussis • It is highly contagious and can be passed from person to person through droplets from the nose and throat of infected individuals when coughing and sneezing • Infants and young children have the highest rates of complications • Infants are the most vulnerable group and almost all deaths from pertussis occur in this age group with most <3 months of age 7 Vaccination against pertussis for pregnant women Photo courtesy of CDC

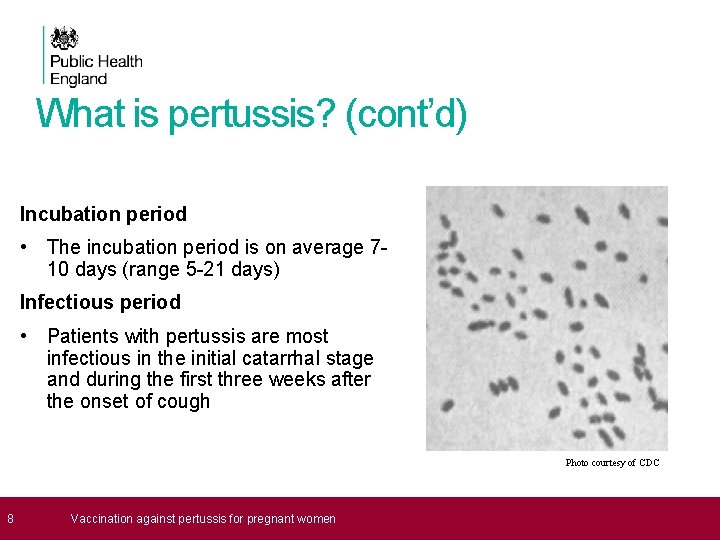
What is pertussis? (cont’d) Incubation period • The incubation period is on average 710 days (range 5 -21 days) Infectious period • Patients with pertussis are most infectious in the initial catarrhal stage and during the first three weeks after the onset of cough Photo courtesy of CDC 8 Vaccination against pertussis for pregnant women

Clinical presentation of pertussis Initial stage Early symptoms are: • similar to those of a cold • can last for one to two weeks before becoming more severe Second or Paroxysmal Stage Characteristic symptoms: • Intense bouts of coughing sometimes referred to as ‘paroxysms’ of coughing • a ‘whoop’ sound with each sharp intake of breath after coughing (may not occur in infants) • Vomiting after coughing 9 Vaccination against pertussis for pregnant women © NHS Scotland/Crown Copyright

Clinical presentation of pertussis (cont’d) Convalescent stage Symptoms: • Slowly become less severe • Generally last 2 -6 weeks but can persist for months © NHS Scotland/Crown Copyright 10 Vaccination against pertussis for pregnant women

Clinical presentation of pertussis in infants and young children Infants may not make the ‘whoop’ sound after coughing, but they may start gagging or gasping and may temporarily stop breathing Young children may also seem to choke or become cyanosed when they have a bout of coughing Photo courtesy of CDC 11 Vaccination against pertussis for pregnant women

Complications in infants and young children Infants and young children are usually most severely affected and more likely to develop severe complications such as: • Pneumonia • Temporary pauses in breathing as a result of severe difficulty with breathing • Weight loss due to excessive vomiting • Seizures or brain damage • Encephalitis (an acute inflammation of the brain) In severe cases pertussis can be fatal in infants 12 Vaccination against pertussis for pregnant women

Complications of pertussis in older children & adults Complications in older children and adults are usually much less serious than those in infants and young children. These may include: • nosebleeds and burst blood vessels in the whites of the eye from intense bouts of coughing • bruised ribs as a result of intense coughing • hernia due to intense coughing • a swollen face • ulcers on the tongue and mouth • ear infections such as otitis media 13 Vaccination against pertussis for pregnant women

Why vaccinate pregnant women against pertussis? 14 Vaccination against pertussis for pregnant women

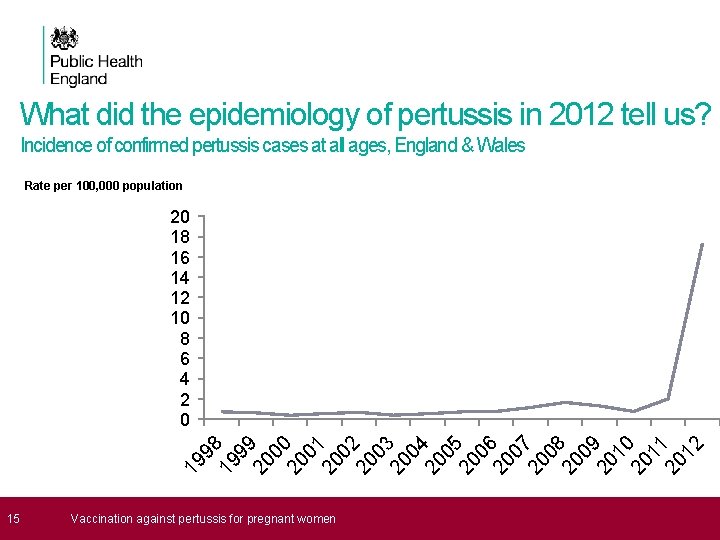
What did the epidemiology of pertussis in 2012 tell us? Incidence of confirmed pertussis cases at all ages, England & Wales Rate per 100, 000 population 19 98 19 99 20 00 20 01 20 02 20 03 20 04 20 05 20 06 20 07 20 08 20 09 20 10 20 11 20 12 20 18 16 14 12 10 8 6 4 2 0 15 Vaccination against pertussis for pregnant women

Age distribution of laboratory confirmed pertussis cases and rate per 100, 000 England Wales 2012 Rate per 100, 000 population Total cases 300 250 9000 8000 7000 6000 5000 4000 3000 2000 1000 0 Total cases 200 150 100 50 rs ye a 15 + ye a 4 9 rs rs hs ye a 10 -1 11 Vaccination against pertussis in pregnancy Age group 1 - m on t m 5 6 - 16 3 - <3 m on th s hs 0

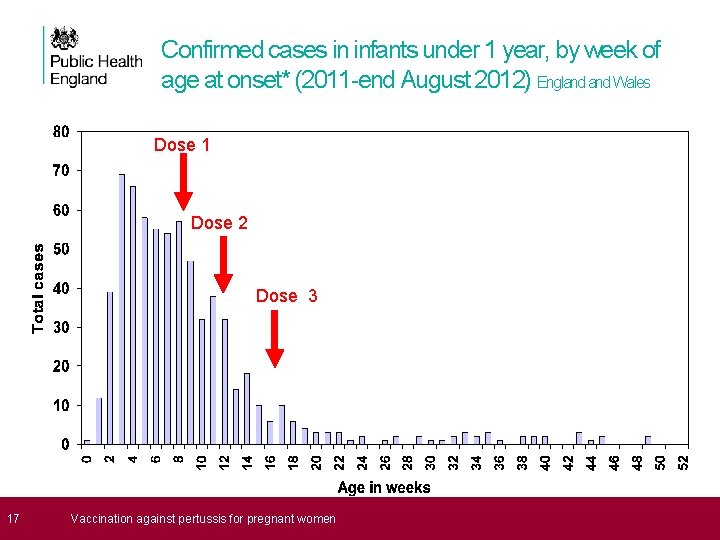
Confirmed cases in infants under 1 year, by week of age at onset* (2011 -end August 2012) England Wales Dose 1 Dose 2 Dose 3 17 * Where provided; specimen date used when onset not Vaccination against pertussis for pregnant women available

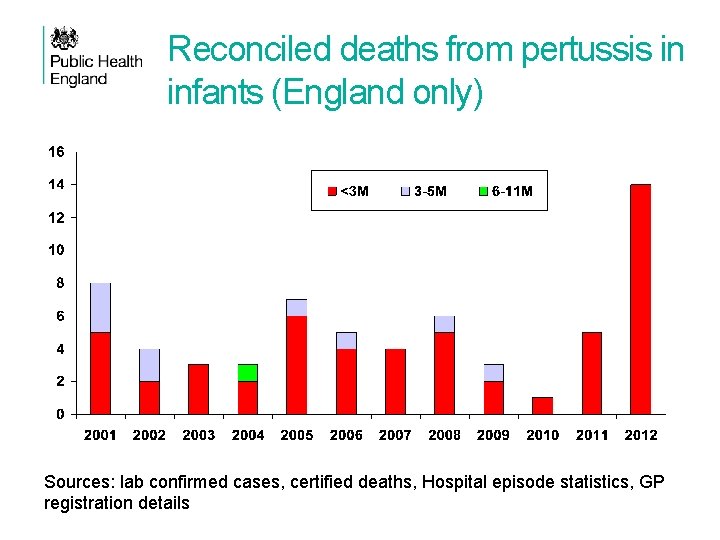
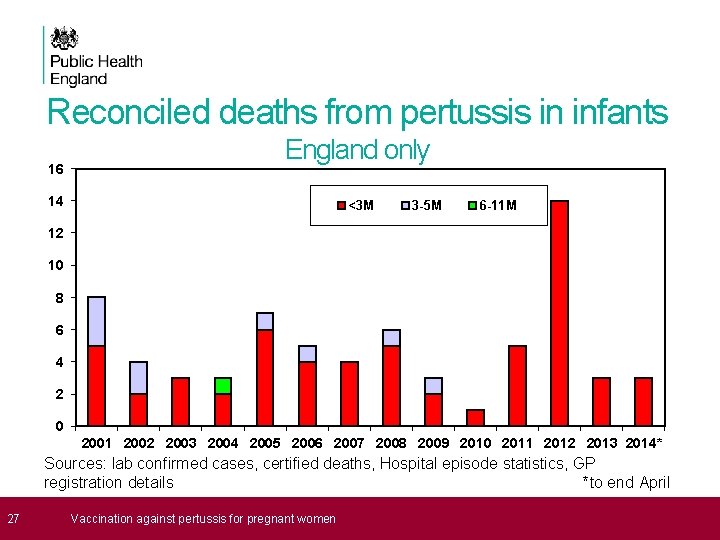
Reconciled deaths from pertussis in infants (England only) Sources: lab confirmed cases, certified deaths, Hospital episode statistics, GP registration details

How can we help prevent pertussis? The childhood vaccination programme • The main strategy for reducing the impact (morbidity and mortality) from pertussis is the current childhood vaccination programme What does this current vaccination programme look like? • Pertussis is part of the infant vaccination programme • 5 -in-1 vaccine (DTa. P/IPV/Hib) is offered to infants at two, three and four months of age. • This protects against pertussis, diphtheria, tetanus, polio and Haemophilus influenzae type b • A booster dose of pertussis containing vaccine is given to children from about three years and four months of age 19 Vaccination against pertussis for pregnant women

Immunity against pertussis • Vaccination against pertussis does not give life-long immunity • Individuals who have previously had pertussis can become re-infected and spread infection to others • This spread of infection is important, particularly in children too young to be vaccinated 20 Vaccination against pertussis for pregnant women

How can we protect children too young to be vaccinated against pertussis? In October 2012, the Department of Health recommended that pregnant women receive • One dose of pertussis containing vaccine from 28 -38 weeks, with the ideal time being 28 -32 weeks gestation • The aim is to boost antibodies in vaccinated women in late pregnancy so that pertussis antibodies are passed from mother to baby • This is considered the best way to provide passive protection to infants in the first weeks of life and offers women a safe way to protect their baby against this serious disease • On the advice of the JCVI, the programme will continue into 2014 21 Vaccination against pertussis for pregnant women © NHS /Crown Copyright

Why vaccinate pregnant women? The immunity acquired by vaccination will be passed across the placenta by antibodies and should help protect the baby in the first few weeks of life, when they are most at risk of serious complications if they become infected with pertussis © NHS Scotland/Crown Copyright 22 Vaccination against pertussis for pregnant women

Why vaccinate pregnant women against pertussis (cont’d)? Helps protect the baby • Babies born to mothers vaccinated at the recommended time during pregnancy should have higher levels of antibodies than those born to unvaccinated mothers, which should help protect the infant until they start receiving their own immunisations Helps protect the mother • Reduces the risk of the mother catching pertussis and passing it on to the young infant 23 Vaccination against pertussis for pregnant women © NHS Scotland/Crown Copyright

Is the pertussis vaccination in pregnancy programme proving effective? 24 Vaccination against pertussis for pregnant women

Collected pertussis vaccine uptake in pregnant women Oct 2012 - March 2014 (England only) т1 но 2 я 1 де 2 к 1 ян 2 в 1 ф 3 ев -1 ма 3 р1 ап 3 р1 ма 3 й 1 ию 3 н 1 ию 3 л 1 ав 3 г-1 се 3 н 1 ок 3 т1 но 3 я 1 де 3 к 1 ян 3 в 1 ф 4 ев -1 ма 4 р14 ок Vaccine Coverage (%) 100 90 80 70 60 50 40 30 20 10 0 Source: Public Health England 25 Vaccination against pertussis for pregnant women Month http: //www. hpa. org. uk/hpr/infections/immunisation. htm

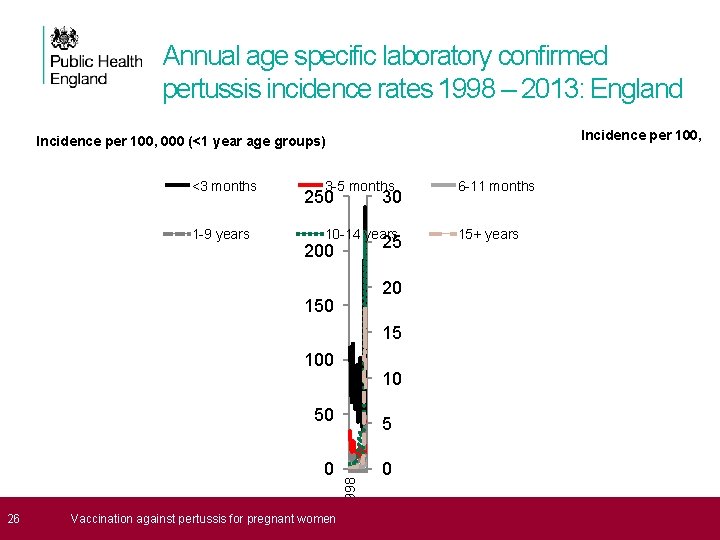
Annual age specific laboratory confirmed pertussis incidence rates 1998 – 2013: England Incidence per 100, 000 (<1 year age groups) <3 months 1 -9 years 3 -5 months 6 -11 months 10 -14 years 15+ years 250 30 25 200 20 15 100 10 50 26 Vaccination against pertussis for pregnant women 1998 0 5 0

Reconciled deaths from pertussis in infants 16 England only 14 <3 M 3 -5 M 6 -11 M 12 10 8 6 4 2 0 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014* Sources: lab confirmed cases, certified deaths, Hospital episode statistics, GP registration details *to end April 27 Vaccination against pertussis for pregnant women

Vaccination against pertussis (whooping cough) - the use of Boostrix-IPV® 28 Vaccination against pertussis for pregnant women

The recommended vaccine: Boostrix-IPV® Brand name: Boostrix-IPV® Generic Name: Diphtheria, Tetanus, Pertussis (acellular, component) and Poliomyelitis (inactivated vaccine) adsorbed (d. Ta. P/IPV) Marketed by Glaxo. Smithkline UK Inactivated (N. B. the vaccine cannot cause pertussis) Presented as a prefilled syringe Image courtesy of Glaxo. Smith. Kline UK 29 Vaccination against pertussis for pregnant women

All staff should be familiar with the BOOSTRIX-IPV packaging Please ensure that you use the correct vaccine and that you accurately record the brand batch number X Ensure that all those administering the vaccine are familiar with the product Images courtesy of Glaxo. Smith. Kline UK 30 Vaccination against pertussis for pregnant women

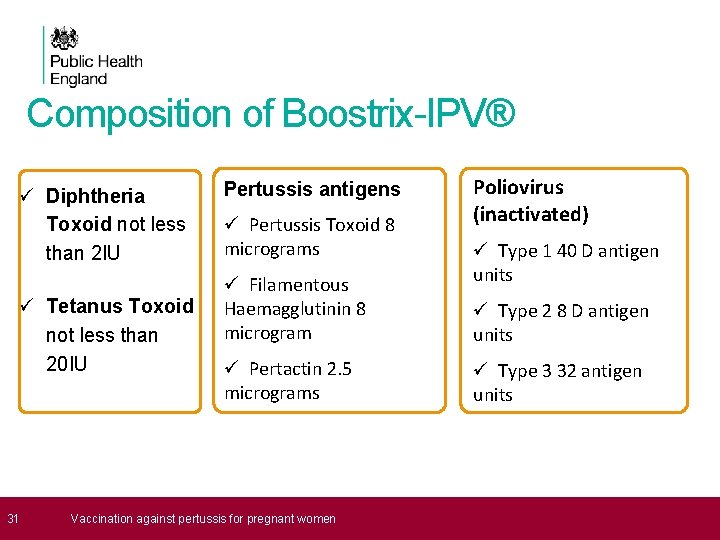
Composition of Boostrix-IPV® ü Diphtheria Toxoid not less than 2 IU ü Tetanus Toxoid not less than 20 IU 31 Pertussis antigens ü Pertussis Toxoid 8 micrograms ü Filamentous Haemagglutinin 8 microgram ü Pertactin 2. 5 micrograms Vaccination against pertussis for pregnant women Poliovirus (inactivated) ü Type 1 40 D antigen units ü Type 2 8 D antigen units ü Type 3 32 antigen units

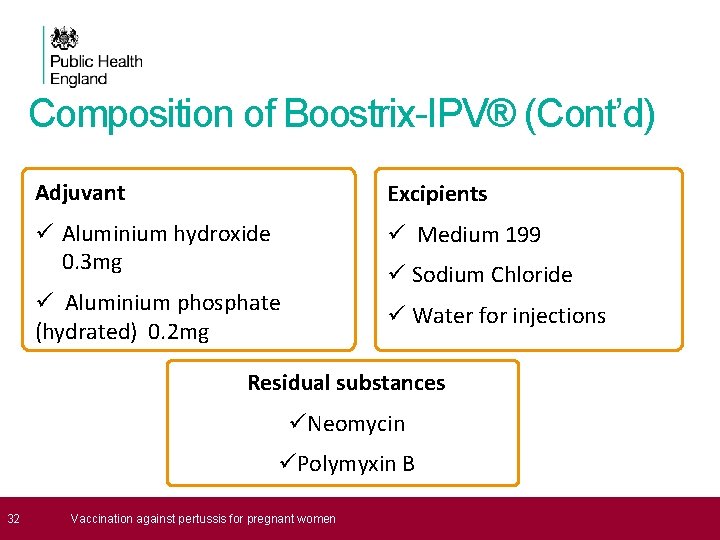
Composition of Boostrix-IPV® (Cont’d) Adjuvant Excipients ü Aluminium hydroxide 0. 3 mg ü Medium 199 ü Sodium Chloride ü Aluminium phosphate (hydrated) 0. 2 mg ü Water for injections Residual substances üNeomycin üPolymyxin B 32 Vaccination against pertussis for pregnant women

Administration of Boostrix-IPV® Licensing: The Green Book states that: “Pertussis-containing vaccines may be given to pregnant women when protection is required without delay. There is no evidence of risk from vaccinating pregnant women or those who are breast-feeding with inactivated viral or bacterial vaccines or toxoids (Plotkin & Orenstein 2004)” 33 Vaccination against pertussis for pregnant women • The vaccine marketing authorisation holder’s Summary of Product Characteristics (SPC) for Boostrix-IPV® states that the vaccine can be used in pregnancy when clearly needed. • In a study of 20, 000 women vaccinated with d. Ta. P/IPV during pregnancy, the MHRA found no evidence of risk • The advice from JCVI to use pertussis containing inactivated vaccines should be followed. There is no evidence of risk to pregnancy or the infant with inactivated vaccines such as Boostrix-IPV®

Administration of Boostrix-IPV® (Cont’d) • The vaccine comes as pre-filled suspension that must be shaken before use to form a white suspension • Given by intramuscular injection into the deltoid • Can be given at the same time as other vaccines such as influenza May only be administered: • Against a prescription written manually or electronically by a registered medical practitioner or other authorised prescriber • Against a Patient Specific Direction • Against a Patient Group Direction 34 Vaccination against pertussis for pregnant women

Contraindications and Precautions Contraindications • A confirmed anaphylactic reaction to a previous dose of diphtheria, tetanus, pertussis or poliomyelitis containing vaccine • A confirmed anaphylactic reaction to any component of the vaccine, including neomycin and/or polymyxin Precautions • Acute illness- defer until recovered • Recent immunisation against pertussis, diphtheria, tetanus and or polio- ensure an interval of 4 weeks • Current neurological deterioration or encephalopathy of unknown origin within seven days of previous immunisation with pertussiscontaining vaccine- follow advice in the Green Book 35 Vaccination against pertussis for pregnant women

Possible adverse reactions Most Common • Erythema (redness), pain, swelling at the injection site Less Common • Pyrexia (>37. 5 C), haematoma, induration, pruritus and warmth at the injection site Uncommon • Swelling of vaccinated limb, pyrexia (>39. 5), chills, pain 36 Vaccination against pertussis for pregnant women

Reporting suspected adverse reactions Yellow card scheme • Voluntary reporting system for suspected adverse reaction to medicines/vaccines • Success depends on early, complete and accurate reporting • Report even if uncertain about whether vaccine caused condition • http: //mhra. gov. uk/yellowcard • See chapter 8 of Green Book for details 37 Vaccination against pertussis for pregnant women

Recommendations • Women who become pregnant again while the programme is in place should be offered immunisation during each pregnancy to maximise transplacental transfer of antibody • One dose of Boostrix-IPV® is recommended for women carrying twins or multiple pregnancies • Vaccination may be offered to new mothers who have never previously been vaccinated against pertussis, up to when their child receives their first vaccination. A single dose of Boostrix-IPV® is recommended in these circumstances • New mothers who have never previously been vaccinated against pertussis are unlikely to have been fully vaccinated against diphtheria, tetanus and polio and should be assessed as to the need for further immunisations with Td/IPV vaccine 38 Vaccination against pertussis for pregnant women

Data management • Health professionals are encouraged to accurately record the brand batch number of the vaccine in relevant data systems following the administration • The delivery date of the baby should also be recorded in the mother’s medical records • This information is essential to allow an assessment of vaccine uptake, safety and to inform any future public health actions, if required • Vaccination against pertussis should be recorded in the women’s medical/maternity records and recorded in the relevant data system • Vaccine uptake of Boostrix-IPV® for pregnant women will be monitored with a monthly data collection though Imm. Form 39 Vaccination against pertussis for pregnant women

Providing longer term protection against pertussis • The protection the infant acquires from the mother by the transfer of antibodies across the placenta is short term • It is very important that parents ensure their infants start their immunisation schedule at the recommended 8 weeks and complete all three doses to receive long lasting protection • Parents should ensure older brothers and/or sisters are up to date with their immunisations 40 Vaccination against pertussis for pregnant women © NHS Scotland/Crown Copyright

The healthcare professionals’ key role To provide clear, concise and accurate information to every pregnant woman regarding vaccination against pertussis Every effort should be made by medical practitioners, midwives and others to maximise the uptake of pertussiscontaining vaccine 41 Vaccination against pertussis for pregnant women

Resources • DH, PHE and NHS England Tripartite Letter (2013) Continuation of temporary programme of pertussis vaccination of pregnant women [link] • NHS Choices (2013). Whooping cough vaccination in pregnancy programme [link] • Public Health England (2013). Immunisation against infectious diseases (Green Book). [link] • Department of Health. Pertussis vaccine in pregnancy programme. [link] • Public Health England (2014) Vaccination against pertussis for pregnant women: an update for health professionals training slide and advice for health professionals document [link] 42 Vaccination against pertussis for pregnant women

Key Message • Infant cases of pertussis have been dramatically reduced by the introduction of the temporary maternal immunisation programme. However, the disease continues to circulate in older age groups • It is important for all health professionals to encourage vaccination in pregnant women from 28 weeks, at the earliest opportunity © NHS Scotland/Crown Copyright 43 Vaccination against pertussis for pregnant women

Acknowledgement This resource was developed by NHS Education for Scotland as a national training template to support the temporary maternal pertussis vaccination programme. Their permission to adapt it for use in England is gratefully acknowledged. Adaption for use in England undertaken by the Immunisation, Hepatitis and Blood Safety Department Public Health England-Colindale 44 Vaccination against pertussis for pregnant women
 Whooping crane population graph
Whooping crane population graph Lecture etymology
Lecture etymology 5 days of sample menus for pregnant women
5 days of sample menus for pregnant women Prayer points for pregnant mothers
Prayer points for pregnant mothers What is vitagen made of
What is vitagen made of Physical violence against women
Physical violence against women Diseases with vaccines
Diseases with vaccines Vaccination schedule in palestine
Vaccination schedule in palestine Sleeping princesses
Sleeping princesses Mandatory vaccination
Mandatory vaccination Vaccination dose
Vaccination dose Spay perry county
Spay perry county Paganini era
Paganini era Vaccination xard
Vaccination xard Epi schedule in pakistan pdf
Epi schedule in pakistan pdf Defaulter vaccination schedule
Defaulter vaccination schedule Lokstallarna jönköping vaccination
Lokstallarna jönköping vaccination Oogenesis defination
Oogenesis defination Poultry vaccination schedule
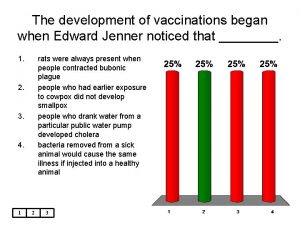
Poultry vaccination schedule Edward jenner
Edward jenner Vaccination bruxelles
Vaccination bruxelles How long do dog periods last
How long do dog periods last 8 weeks pregnant ultrasound
8 weeks pregnant ultrasound Was elizabeth proctor pregnant
Was elizabeth proctor pregnant Molly fish
Molly fish Odds of getting pregnant
Odds of getting pregnant Antenatal investigations
Antenatal investigations How long does a dog stay in heat
How long does a dog stay in heat Scientific name for sugar glider
Scientific name for sugar glider Numberblocks pregnant
Numberblocks pregnant Sperm volume normal range
Sperm volume normal range Dogmatism fallacy definition
Dogmatism fallacy definition Correcting windswept foal
Correcting windswept foal Richa shukla pregnant
Richa shukla pregnant Dr julia turner
Dr julia turner Can you take ventolin while pregnant
Can you take ventolin while pregnant Semen analysis normal report
Semen analysis normal report Are mosquito fish guppies
Are mosquito fish guppies Period cramps in early pregnancy
Period cramps in early pregnancy Richa shukla pregnant
Richa shukla pregnant Holometablous
Holometablous The crucible unit test review
The crucible unit test review Morrissey cough impulse
Morrissey cough impulse Dr veronica white
Dr veronica white Hengityspalje
Hengityspalje