Unit 7 Chemical Equations Chemical Equations Reactants the



- Slides: 21

Unit 7 Chemical Equations

Chemical Equations • Reactants – the substances that exist before a chemical change (or reaction) takes place. • Products – the new substance(s) that are formed during the chemical changes. • CHEMICAL EQUATION indicates the reactants and products of a reaction. REACTANTS PRODUCTS

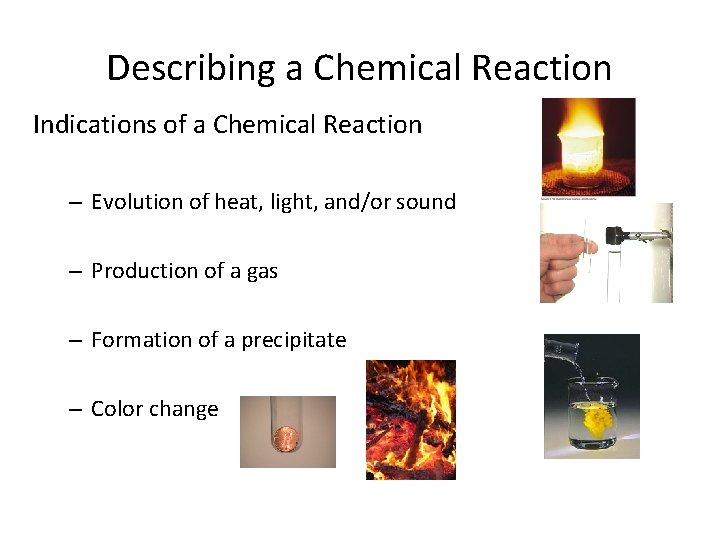
Describing a Chemical Reaction Indications of a Chemical Reaction – Evolution of heat, light, and/or sound – Production of a gas – Formation of a precipitate – Color change

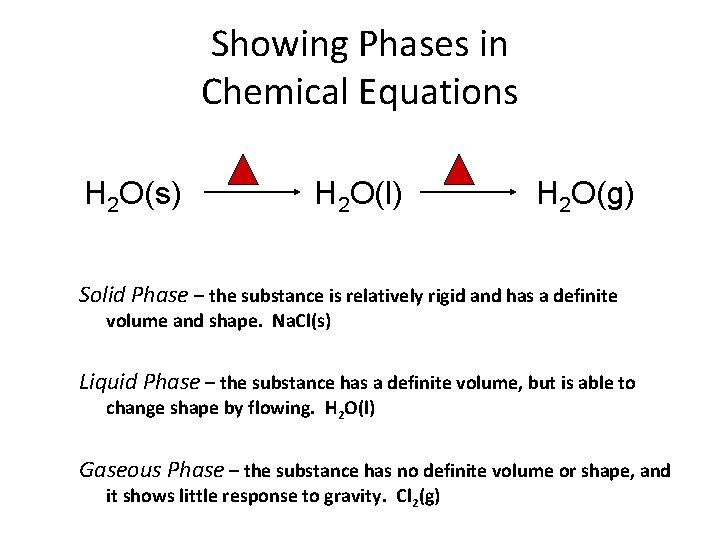
Showing Phases in Chemical Equations H 2 O(s) H 2 O(l) H 2 O(g) Solid Phase – the substance is relatively rigid and has a definite volume and shape. Na. Cl(s) Liquid Phase – the substance has a definite volume, but is able to change shape by flowing. H 2 O(l) Gaseous Phase – the substance has no definite volume or shape, and it shows little response to gravity. Cl 2(g)

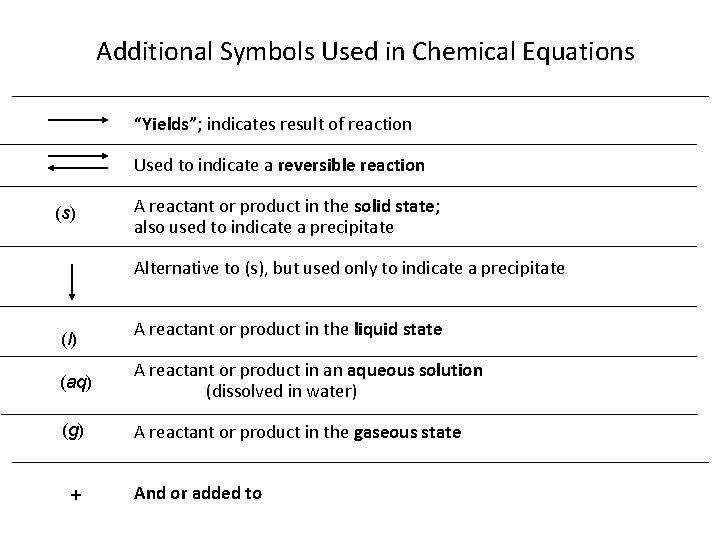
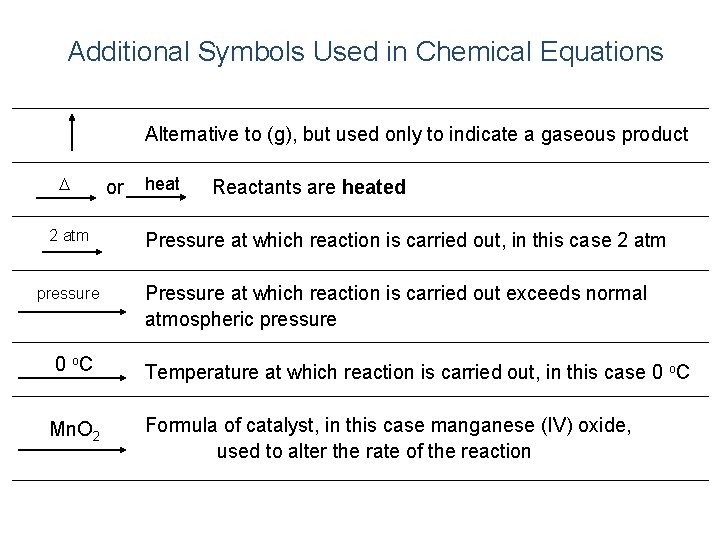
Additional Symbols Used in Chemical Equations “Yields”; indicates result of reaction Used to indicate a reversible reaction (s) A reactant or product in the solid state; also used to indicate a precipitate Alternative to (s), but used only to indicate a precipitate (l) A reactant or product in the liquid state (aq) A reactant or product in an aqueous solution (dissolved in water) (g) A reactant or product in the gaseous state + And or added to

Additional Symbols Used in Chemical Equations Alternative to (g), but used only to indicate a gaseous product D 2 atm pressure or heat Reactants are heated Pressure at which reaction is carried out, in this case 2 atm Pressure at which reaction is carried out exceeds normal atmospheric pressure 0 o. C Temperature at which reaction is carried out, in this case 0 o. C Mn. O 2 Formula of catalyst, in this case manganese (IV) oxide, used to alter the rate of the reaction

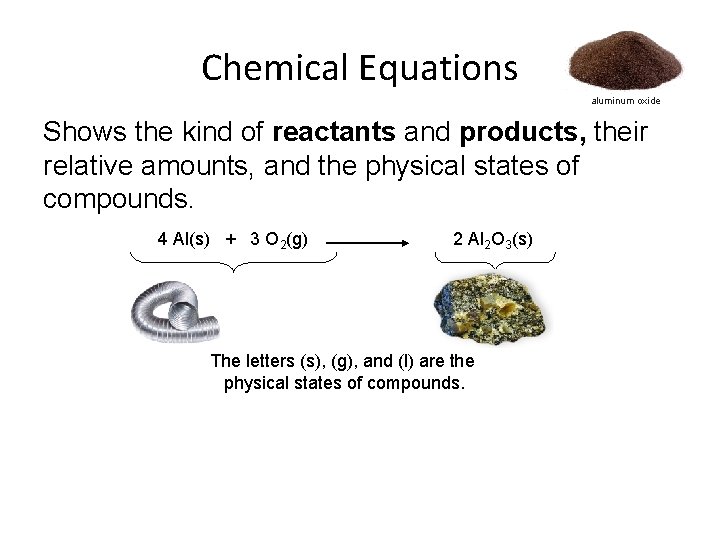
Chemical Equations aluminum oxide Shows the kind of reactants and products, their relative amounts, and the physical states of compounds. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) The letters (s), (g), and (l) are the physical states of compounds.

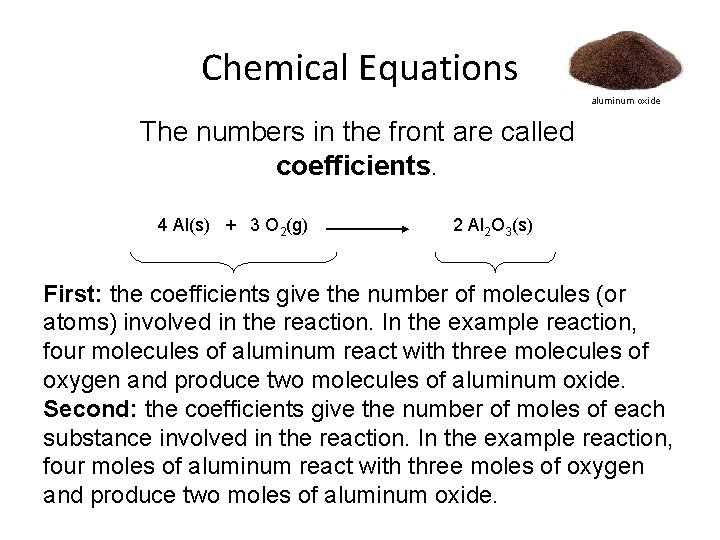
Chemical Equations aluminum oxide The numbers in the front are called coefficients. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) First: the coefficients give the number of molecules (or atoms) involved in the reaction. In the example reaction, four molecules of aluminum react with three molecules of oxygen and produce two molecules of aluminum oxide. Second: the coefficients give the number of moles of each substance involved in the reaction. In the example reaction, four moles of aluminum react with three moles of oxygen and produce two moles of aluminum oxide.

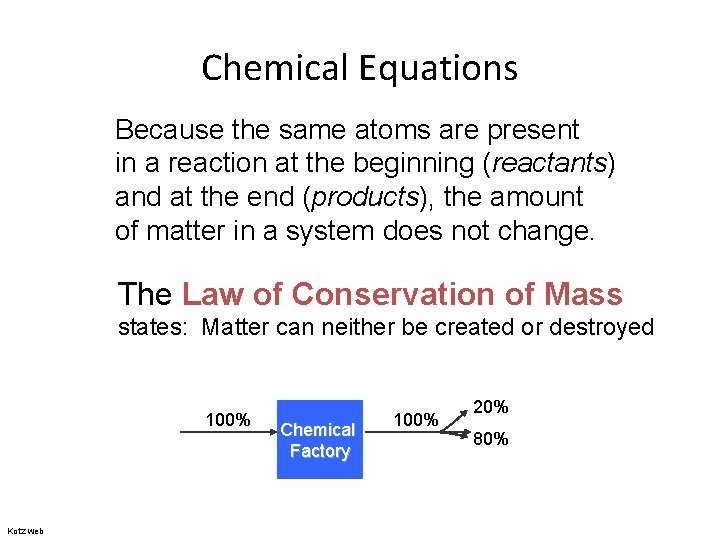
Chemical Equations Because the same atoms are present in a reaction at the beginning (reactants) and at the end (products), the amount of matter in a system does not change. The Law of Conservation of Mass states: Matter can neither be created or destroyed 100% Kotz web Chemical Factory 100% 20% 80%

Chemical Equations Because of the principle of the conservation of mass, mass all equations must be balanced. There must be the same number of atoms and the same kind of atoms on both sides of equation. Reactants = Products Lavoisier, 1788 What a ladies man!

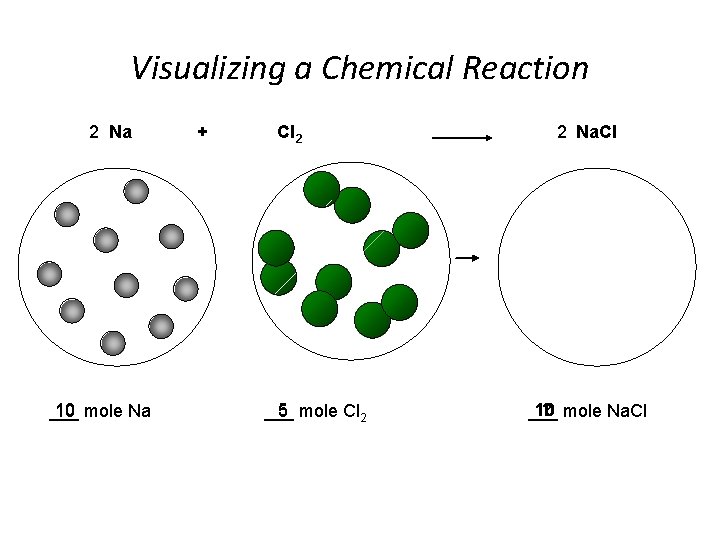
Visualizing a Chemical Reaction 2 Na 10 mole Na ___ + Cl 2 5 mole Cl 2 ___ 2 Na. Cl 10 ? mole Na. Cl ___

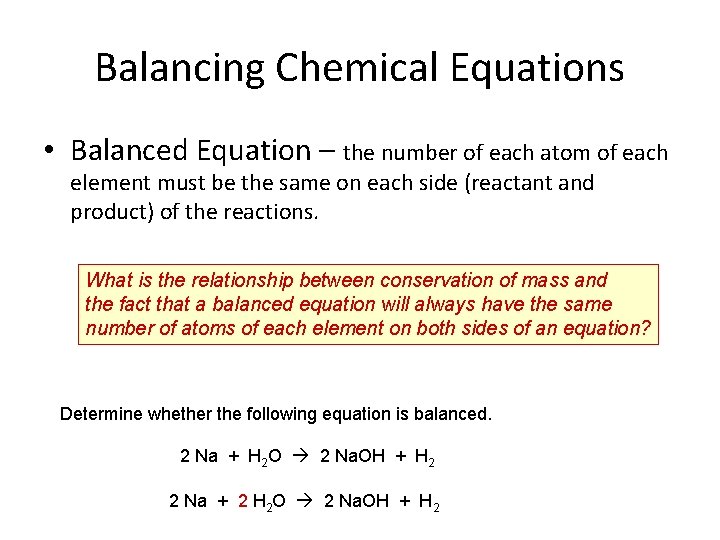
Balancing Chemical Equations • Balanced Equation – the number of each atom of each element must be the same on each side (reactant and product) of the reactions. What is the relationship between conservation of mass and the fact that a balanced equation will always have the same number of atoms of each element on both sides of an equation? Determine whether the following equation is balanced. 2 Na + H 2 O 2 Na. OH + H 2 2 Na + 2 H 2 O 2 Na. OH + H 2

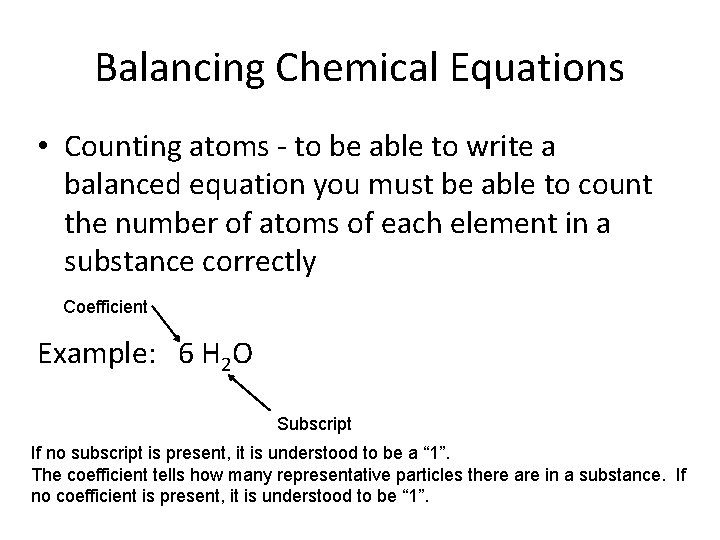
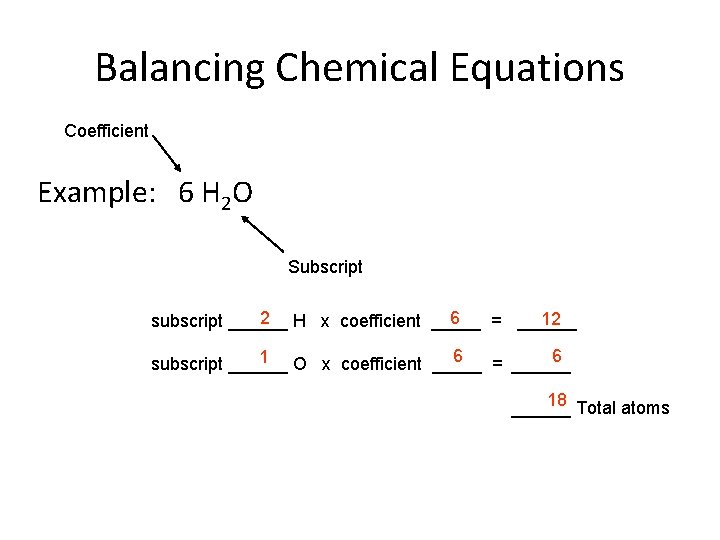
Balancing Chemical Equations • Counting atoms - to be able to write a balanced equation you must be able to count the number of atoms of each element in a substance correctly Coefficient Example: 6 H 2 O Subscript If no subscript is present, it is understood to be a “ 1”. The coefficient tells how many representative particles there are in a substance. If no coefficient is present, it is understood to be “ 1”.

Balancing Chemical Equations Coefficient Example: 6 H 2 O Subscript 2 H x coefficient _____ 6 12 subscript ______ = ______ 6 6 1 O x coefficient _____ subscript ______ = ______ 18 Total atoms ______

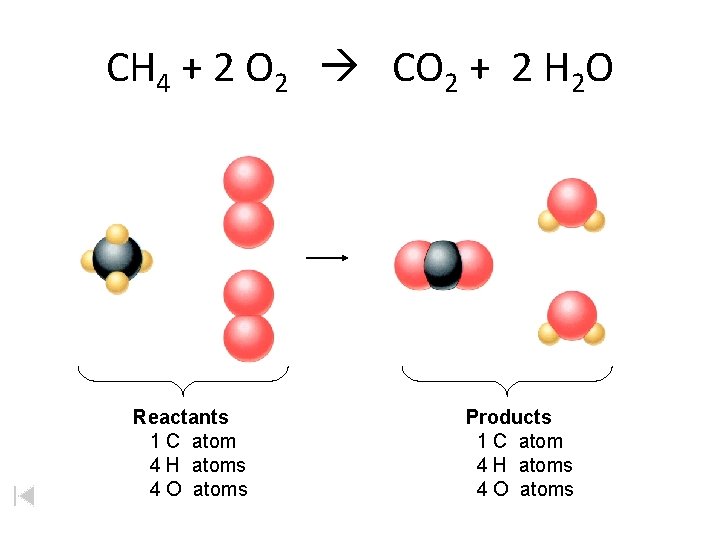
CH 4 + 2 O 2 CO 2 + 2 H 2 O Reactants 1 C atom 4 H atoms 4 O atoms Products 1 C atom 4 H atoms 4 O atoms

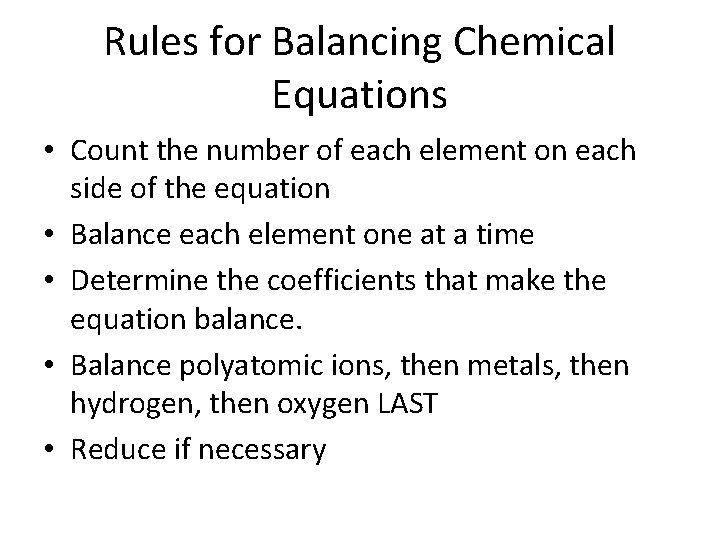
Rules for Balancing Chemical Equations • Count the number of each element on each side of the equation • Balance each element one at a time • Determine the coefficients that make the equation balance. • Balance polyatomic ions, then metals, then hydrogen, then oxygen LAST • Reduce if necessary

Balancing Chemical Equations Watch youtube video lessons for balancing equations by clicking the links below. https: //www. youtube. com/watch? v=gskm-df. Kv 5 g https: //www. youtube. com/watch? v=_B 735 tur. Do. M Watch youtube video lessons for completing worksheets. Review: Counting Atoms in Compounds Review: Forming and Naming Compounds Which Equations are Balanced? Balancing Equations Word Equations

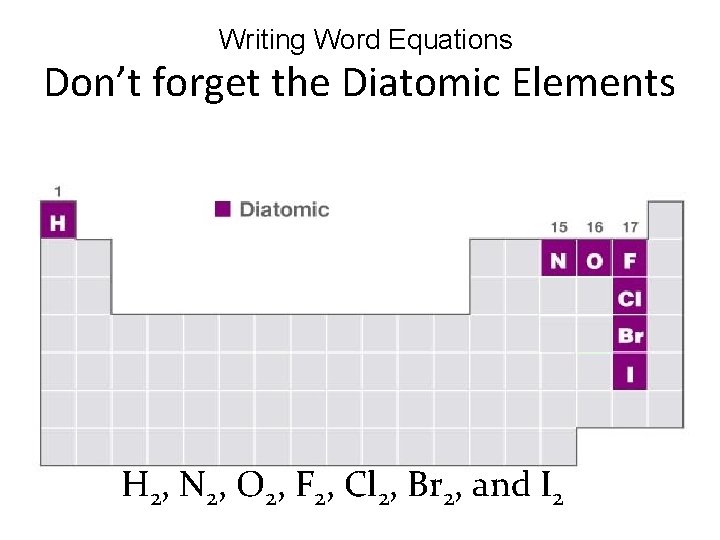
Writing Word Equations Don’t forget the Diatomic Elements H 2, N 2, O 2, F 2, Cl 2, Br 2, and I 2

Writing Word Equations See any diatomic? ? ? • • • Cl 2 Diatomic Br 2 Diatomic Mg H H 2 Diatomic Ca S S

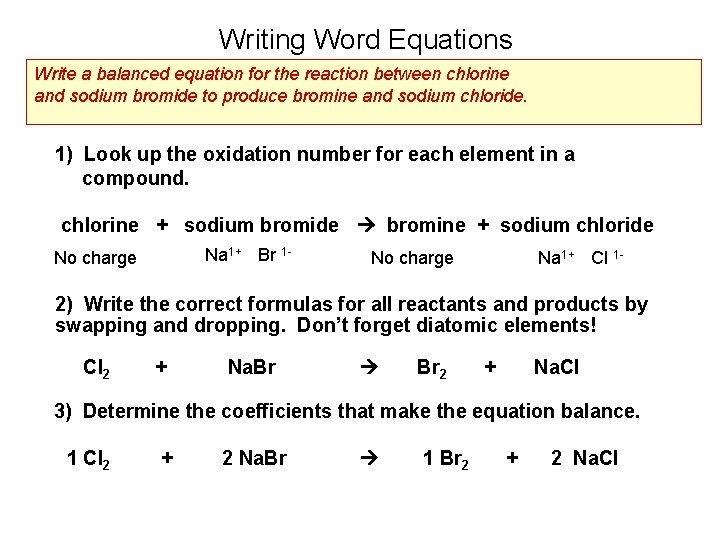
Writing Word Equations Write a balanced equation for the reaction between chlorine and sodium bromide to produce bromine and sodium chloride. 1) Look up the oxidation number for each element in a compound. chlorine + sodium bromide bromine + sodium chloride Na 1+ Br 1 - No charge Na 1+ Cl 1 - 2) Write the correct formulas for all reactants and products by swapping and dropping. Don’t forget diatomic elements! Cl 2 + Na. Br Br 2 + Na. Cl 3) Determine the coefficients that make the equation balance. 1 Cl 2 + 2 Na. Br 1 Br 2 + 2 Na. Cl

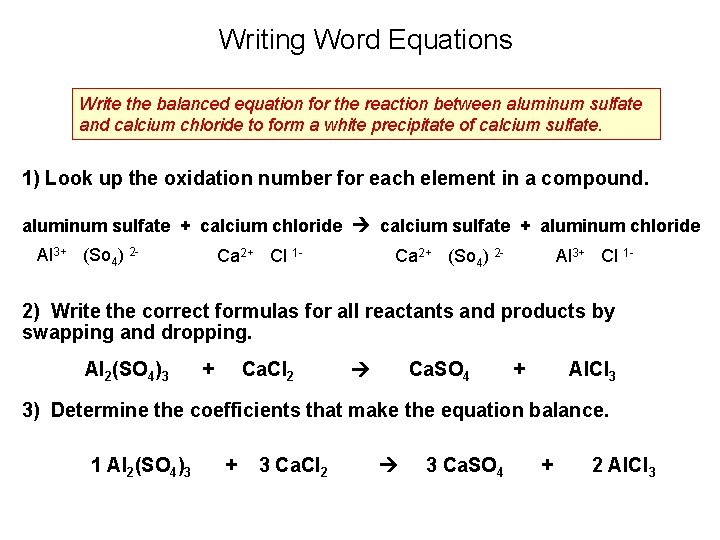
Writing Word Equations Write the balanced equation for the reaction between aluminum sulfate and calcium chloride to form a white precipitate of calcium sulfate. 1) Look up the oxidation number for each element in a compound. aluminum sulfate + calcium chloride calcium sulfate + aluminum chloride Al 3+ (So 4) 2 - Ca 2+ Cl 1 - Ca 2+ (So 4) 2 - Al 3+ Cl 1 - 2) Write the correct formulas for all reactants and products by swapping and dropping. Al 2(SO 4)3 + Ca. Cl 2 Ca. SO 4 + Al. Cl 3 3) Determine the coefficients that make the equation balance. 1 Al 2(SO 4)3 + 3 Ca. Cl 2 3 Ca. SO 4 + 2 Al. Cl 3