THIN FILM CHARACTERIZATION TECHNIQUES OPTICAL TECHNIQUES EP 727












- Slides: 43

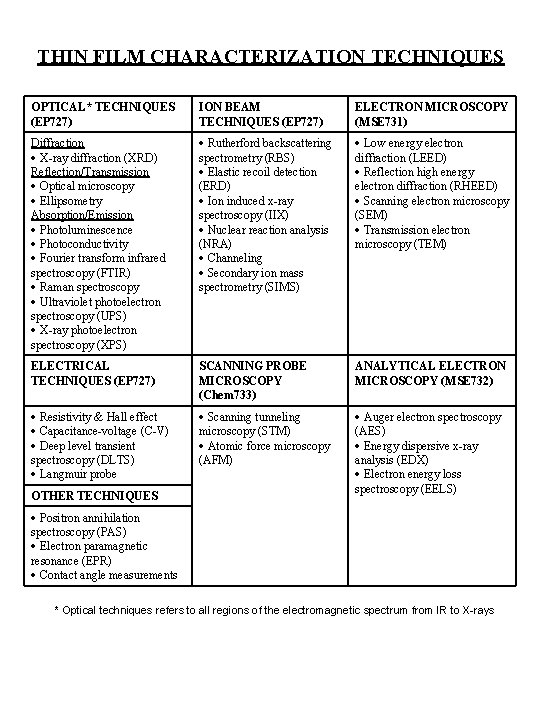
THIN FILM CHARACTERIZATION TECHNIQUES OPTICAL* TECHNIQUES (EP 727) ION BEAM TECHNIQUES (EP 727) ELECTRON MICROSCOPY (MSE 731) Diffraction X-ray diffraction (XRD) Reflection/Transmission Optical microscopy Ellipsometry Absorption/Emission Photoluminescence Photoconductivity Fourier transform infrared spectroscopy (FTIR) Raman spectroscopy Ultraviolet photoelectron spectroscopy (UPS) X-ray photoelectron spectroscopy (XPS) Rutherford backscattering spectrometry (RBS) Elastic recoil detection (ERD) Ion induced x-ray spectroscopy (IIX) Nuclear reaction analysis (NRA) Channeling Secondary ion mass spectrometry (SIMS) Low energy electron diffraction (LEED) Reflection high energy electron diffraction (RHEED) Scanning electron microscopy (SEM) Transmission electron microscopy (TEM) ELECTRICAL TECHNIQUES (EP 727) SCANNING PROBE MICROSCOPY (Chem 733) ANALYTICAL ELECTRON MICROSCOPY (MSE 732) Resistivity & Hall effect Capacitance-voltage (C-V) Deep level transient spectroscopy (DLTS) Langmuir probe Scanning tunneling microscopy (STM) Atomic force microscopy (AFM) Auger electron spectroscopy (AES) Energy dispersive x-ray analysis (EDX) Electron energy loss spectroscopy (EELS) OTHER TECHNIQUES Positron annihilation spectroscopy (PAS) Electron paramagnetic resonance (EPR) Contact angle measurements * Optical techniques refers to all regions of the electromagnetic spectrum from IR to X-rays

THIN FILM CHARACTERIZATION TECHNIQUES • Mass, energy, or wavelength of reflected or transmitted particles are measured • Used to determine film structure, composition, thickness, etc. Incident electrons, ions, or photons Reflected or emitted particles Sample Transmitted particles

Film Structure Direct Imaging SPM Diffraction (electrons, x-rays) LEED RHEED XRD Electron Microscopy

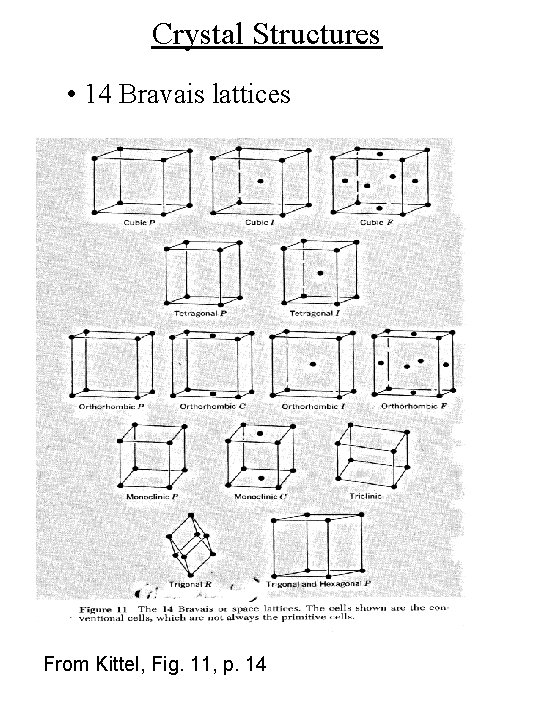
Crystal Structures • 14 Bravais lattices From Kittel, Fig. 11, p. 14

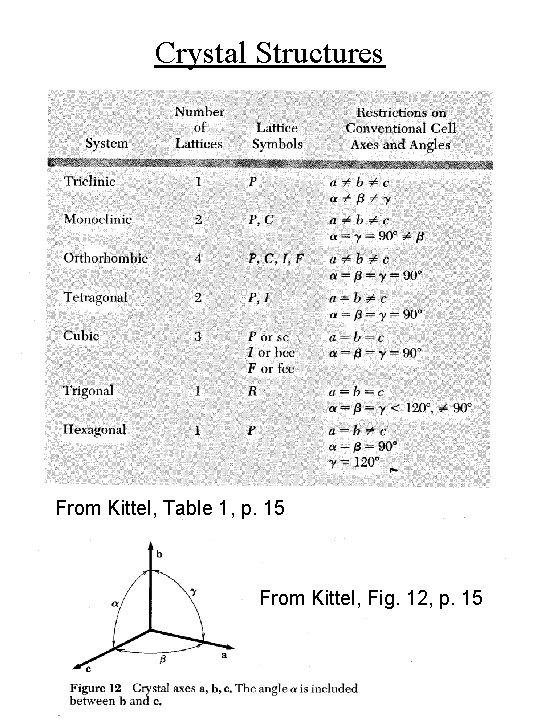
Crystal Structures From Kittel, Table 1, p. 15 From Kittel, Fig. 12, p. 15

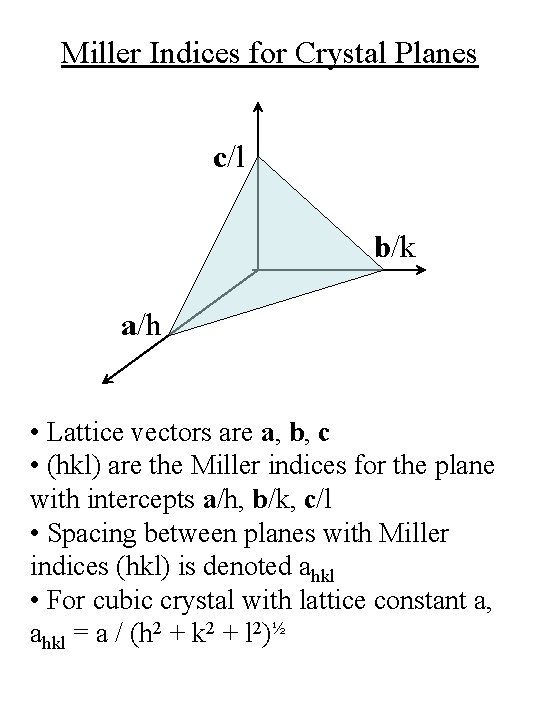
Miller Indices for Crystal Planes c/l b/k a/h • Lattice vectors are a, b, c • (hkl) are the Miller indices for the plane with intercepts a/h, b/k, c/l • Spacing between planes with Miller indices (hkl) is denoted ahkl • For cubic crystal with lattice constant a, ahkl = a / (h 2 + k 2 + l 2)½

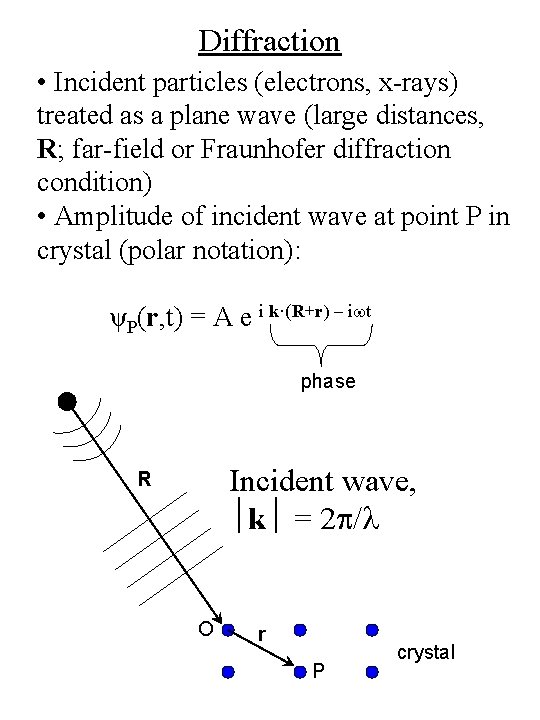
Diffraction • Incident particles (electrons, x-rays) treated as a plane wave (large distances, R; far-field or Fraunhofer diffraction condition) • Amplitude of incident wave at point P in crystal (polar notation): ψP(r, t) = A e i k·(R+r) – iwt phase Incident wave, k = 2 p/l R O r P crystal

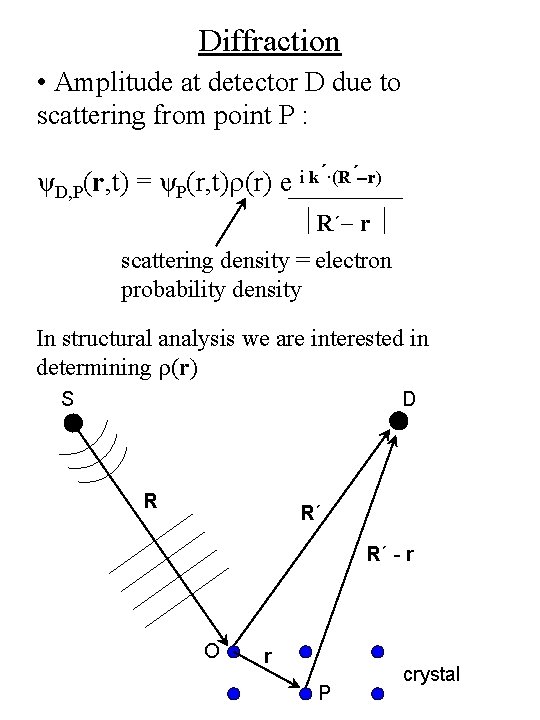
Diffraction • Amplitude at detector D due to scattering from point P : ψD, P(r, t) = ψP(r, t)r(r) e i k´·(R´-r) R´- r scattering density = electron probability density In structural analysis we are interested in determining r(r) S D R R´ R´ - r O r P crystal

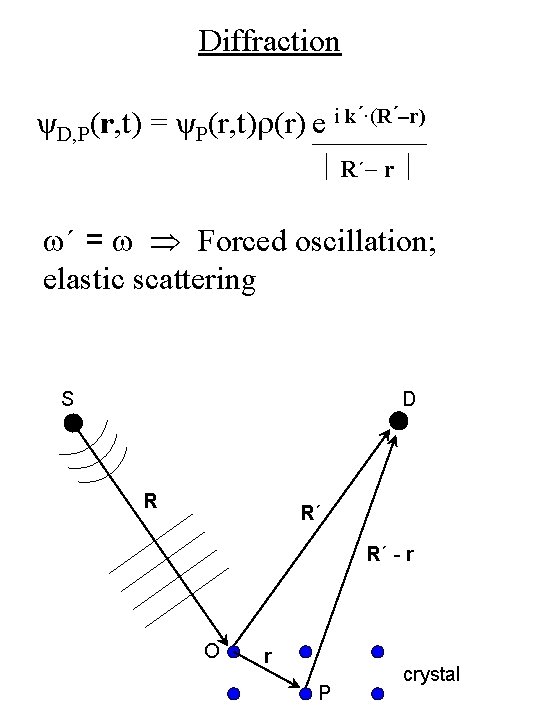
Diffraction ψD, P(r, t) = ψP(r, t)r(r) e i k´·(R´-r) R´- r w´ = w Forced oscillation; elastic scattering S D R R´ R´ - r O r P crystal

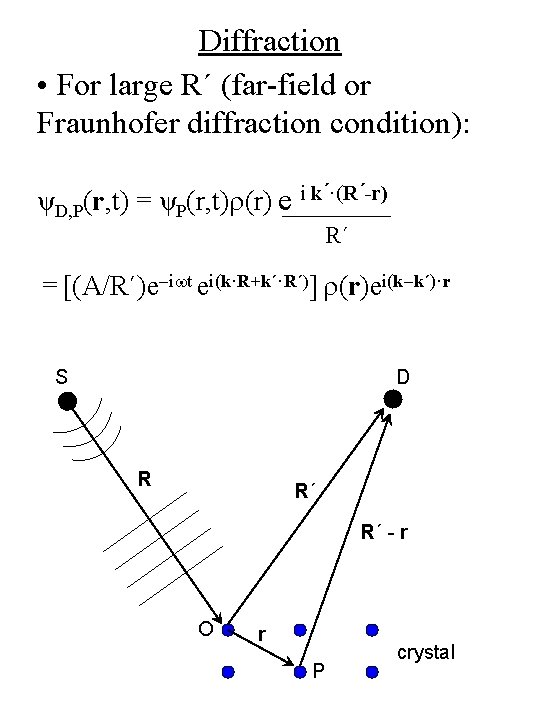
Diffraction • For large R´ (far-field or Fraunhofer diffraction condition): ψD, P(r, t) = ψP(r, t)r(r) e i k´·(R´-r) R´ = [(A/R´)e-iwt ei(k·R+k´·R´)] r(r)ei(k-k´)·r S D R R´ R´ - r O r P crystal

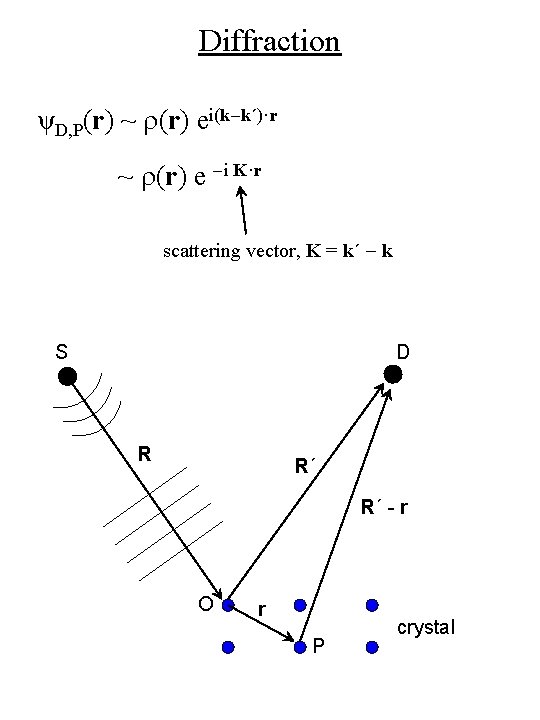
Diffraction ψD, P(r) ~ r(r) ei(k-k´)·r ~ r(r) e -i K·r scattering vector, K = k´ - k S D R R´ R´ - r O r P crystal

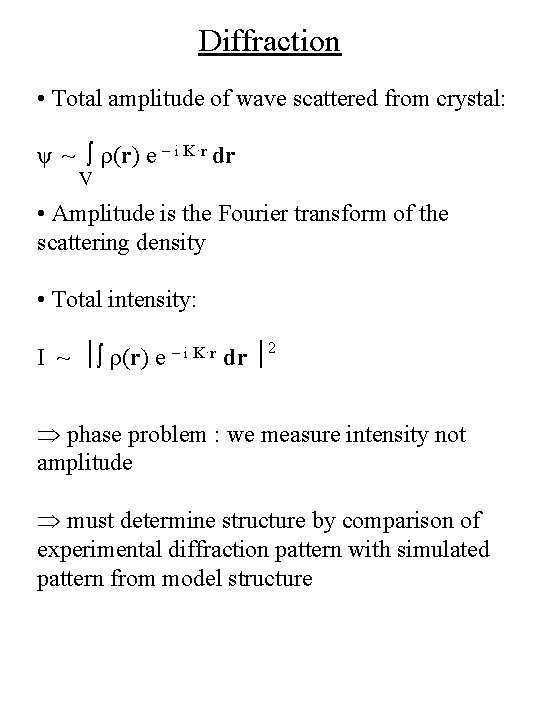
Diffraction • Total amplitude of wave scattered from crystal: ψ ~ ∫ r(r) e - i K·r dr V • Amplitude is the Fourier transform of the scattering density • Total intensity: I ~ ∫ r(r) e - i K·r dr 2 phase problem : we measure intensity not amplitude must determine structure by comparison of experimental diffraction pattern with simulated pattern from model structure

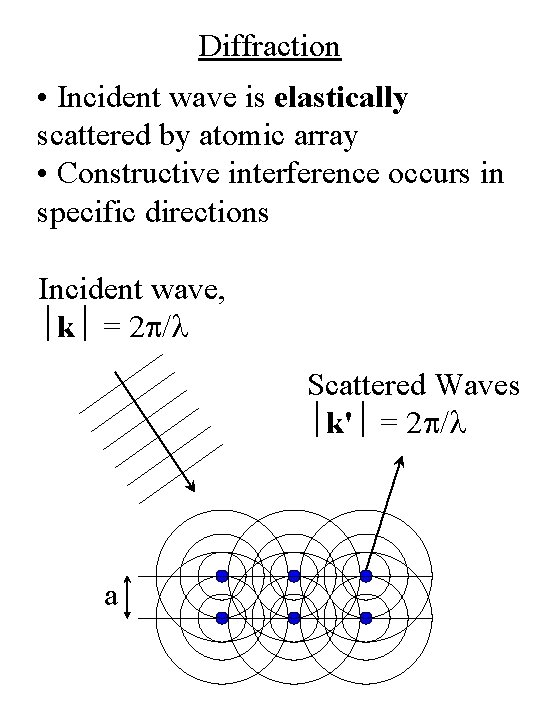
Diffraction • Incident wave is elastically scattered by atomic array • Constructive interference occurs in specific directions Incident wave, k = 2 p/l Scattered Waves k' = 2 p/l a

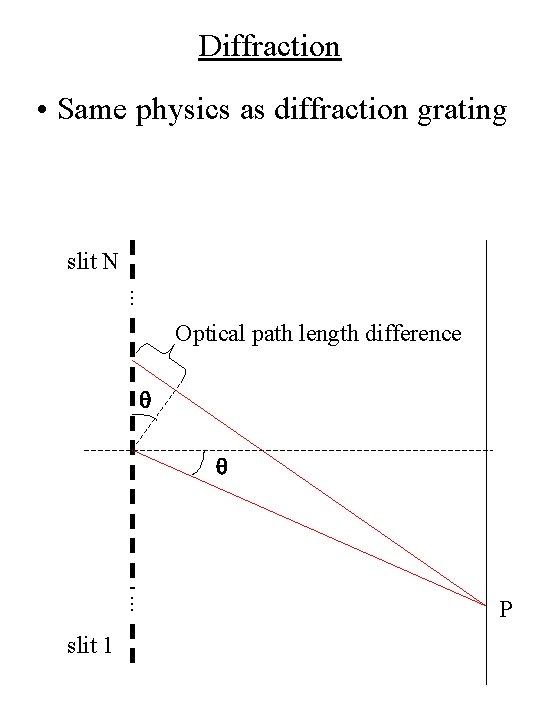
Diffraction • Same physics as diffraction grating slit N. . . Optical path length difference q q . . . slit 1 P

Diffraction • Constructive interference occurs when: Path length difference = ml 2 ahkl sinq = ml (Bragg condition) m = diffraction order = 1, 2, 3, … q’s that satisfy the Bragg condition are called Bragg angles, qb k ahkl k' k q k' A B r

Diffraction Note : q defined relative to surface plane, not the surface normal (as in optics) k ahkl k' k q k' A B r

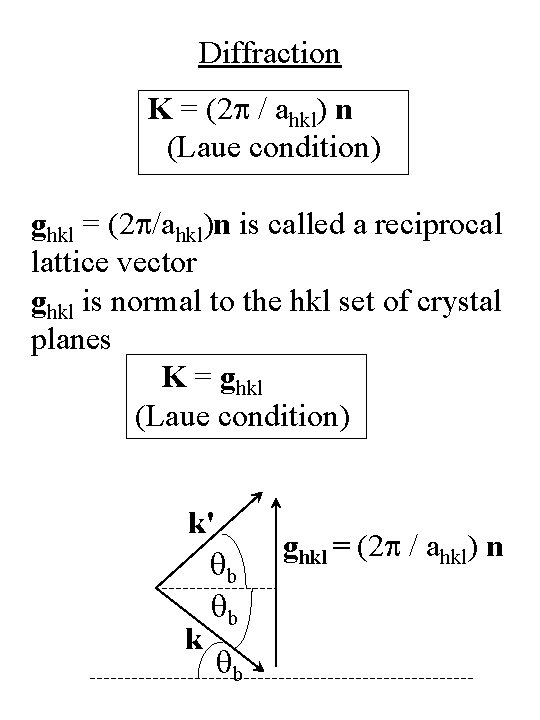
Diffraction • Phase difference between plane waves reflected from atoms such as A and B is Df = (k' – k) • r = K • r Constructive interference occurs when n = unit vector Df = 2 p along surface normal or K = (2 p/ahkl) n (Laue condition) k ahkl k' k q k' A B r

Diffraction K = (2 p / ahkl) n (Laue condition) ghkl = (2 p/ahkl)n is called a reciprocal lattice vector ghkl is normal to the hkl set of crystal planes K = ghkl (Laue condition) k' qb qb k qb ghkl = (2 p / ahkl) n

Miller Indices c/l ghkl b/k a/h • Lattice vectors are a, b, c • Reciprocal lattice vectors are a*, b*, c* ghkl = (ha* + kb* + lc*) is orthogonal to hkl plane; h, k, l are integers

Diffraction • The set of all ghkl points is called reciprocal space • ghkl defines a point in reciprocal space for each set of crystal planes in real space with spacing ahkl ghkl Origin, O

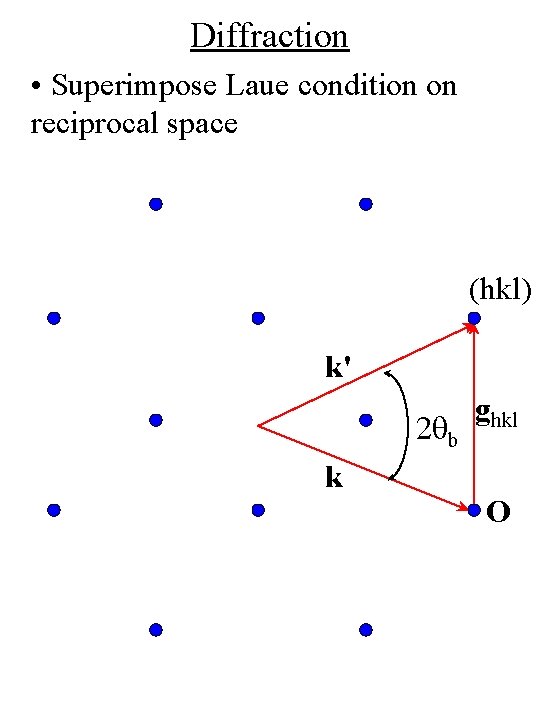
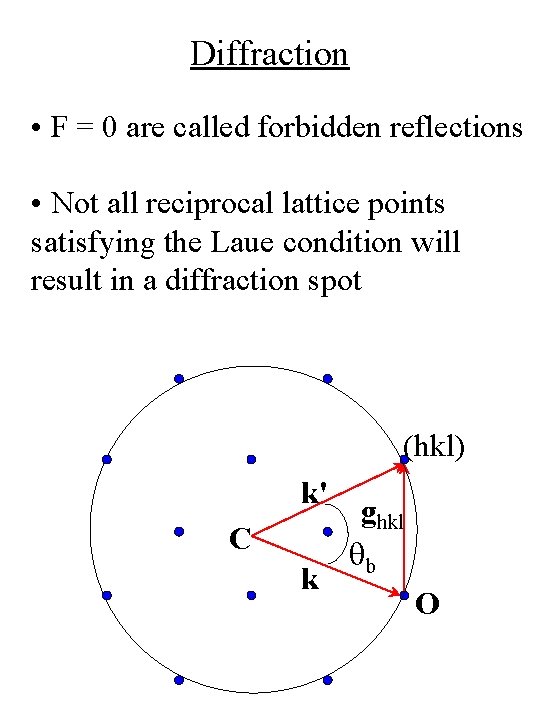
Diffraction • Superimpose Laue condition on reciprocal space (hkl) k' 2 qb k ghkl O

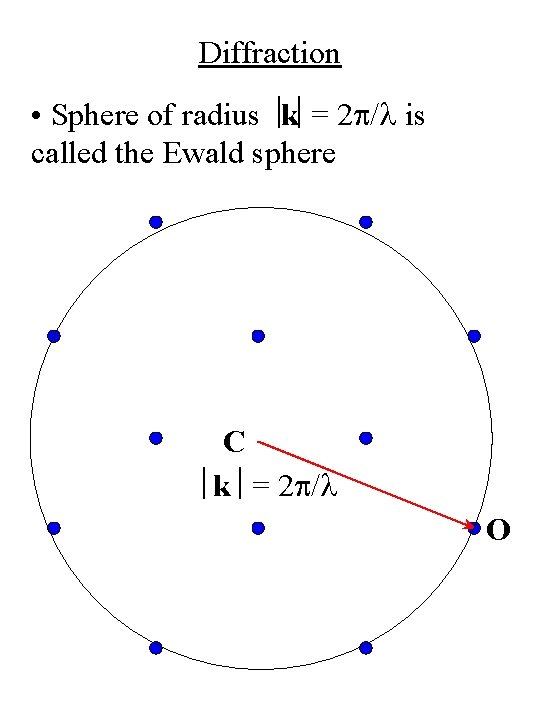
Diffraction • Sphere of radius k = 2 p/l is called the Ewald sphere C k = 2 p/l O

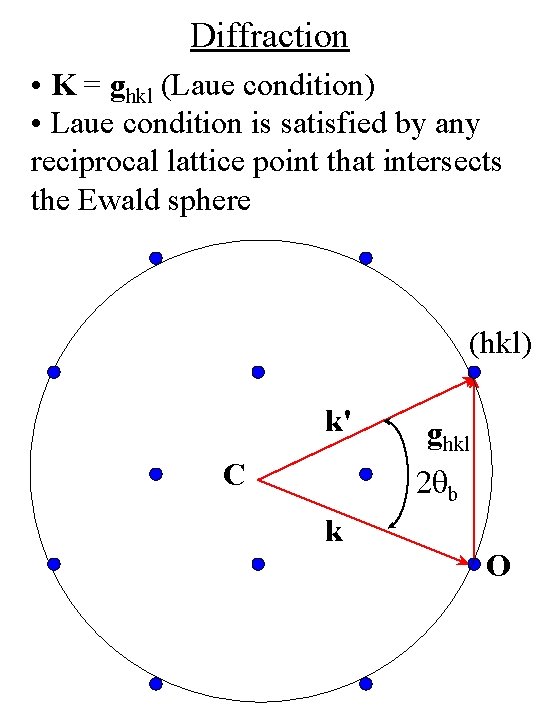
Diffraction • K = ghkl (Laue condition) • Laue condition is satisfied by any reciprocal lattice point that intersects the Ewald sphere (hkl) k' C ghkl 2 qb k O

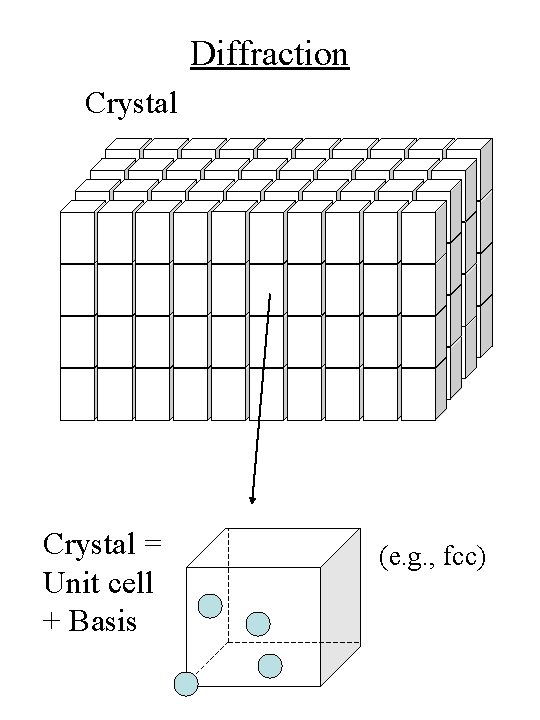
Diffraction Crystal = Unit cell + Basis (e. g. , fcc)

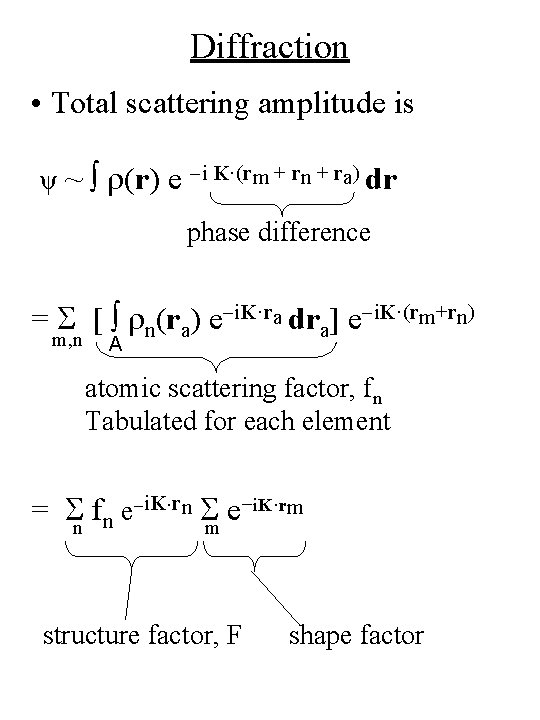
Diffraction r = rm + rn + ra position of mth unit cell nth position of atom in each unit cell ra rn rm O position vector from centre of charge distribution within each atom

Diffraction • Total scattering amplitude is ψ ~ ∫ r(r) e -i K·(rm + rn + ra) dr phase difference = S [ ∫ rn(ra) e-i. K·ra dra] e-i. K·(rm+rn) m, n A atomic scattering factor, fn Tabulated for each element = Sn fn e-i. K·rn Sm e-i. K·rm structure factor, F shape factor

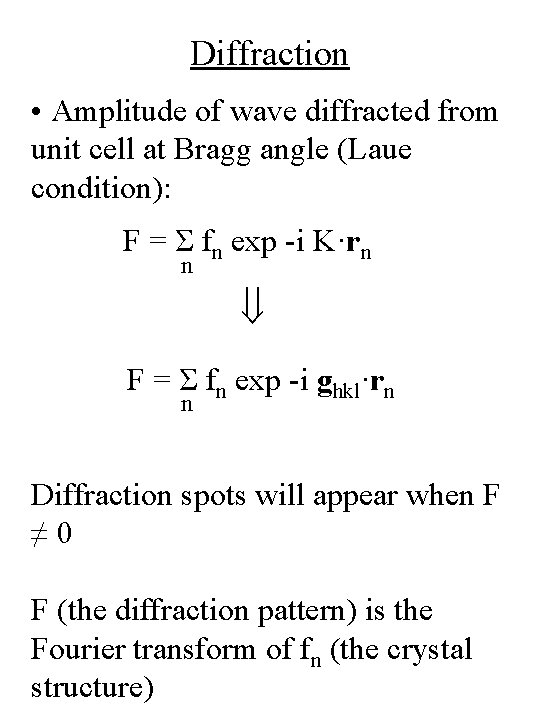
Diffraction • Amplitude of wave scattered from a single unit cell: position of atom n in unit cell Structure factor F = Σ fn exp -i. K·rn n phase difference scattering factor from atom n summation over n atoms in unit cell Assumes electrons only scatter once (kinematic approximation)

Diffraction • Amplitude of wave diffracted from unit cell at Bragg angle (Laue condition): F = Σ fn exp -i K·rn n F = Σ fn exp -i ghkl·rn n Diffraction spots will appear when F ≠ 0 F (the diffraction pattern) is the Fourier transform of fn (the crystal structure)

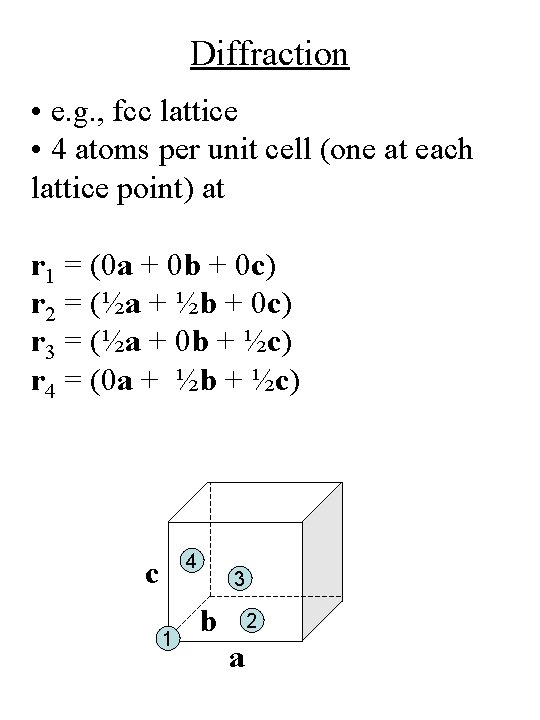
Diffraction • e. g. , fcc lattice • 4 atoms per unit cell (one at each lattice point) at r 1 = (0 a + 0 b + 0 c) r 2 = (½a + ½b + 0 c) r 3 = (½a + 0 b + ½c) r 4 = (0 a + ½b + ½c) 4 c 1 3 b 2 a

Diffraction F = Σ fn exp -i ghkl·rn n ghkl = (ha*+ kb*+ lc*) r 1 = (0 a + 0 b + 0 c) r 2 = (½a + ½b + 0 c) r 3 = (½a + 0 b + ½c) r 4 = (0 a + ½b + ½c) F = f [ 1 + e-ip(h+k) + e-ip(h+l) + e-ip(k+l) ] F= 0 if h, k, l are mixed (even & odd) integers 4 f if h, k, l are unmixed (all even or all odd) e. g. , (400) → F = 4 f e. g. , (211) → F = 0

Diffraction • F = 0 are called forbidden reflections • Not all reciprocal lattice points satisfying the Laue condition will result in a diffraction spot (hkl) k' C k ghkl qb O

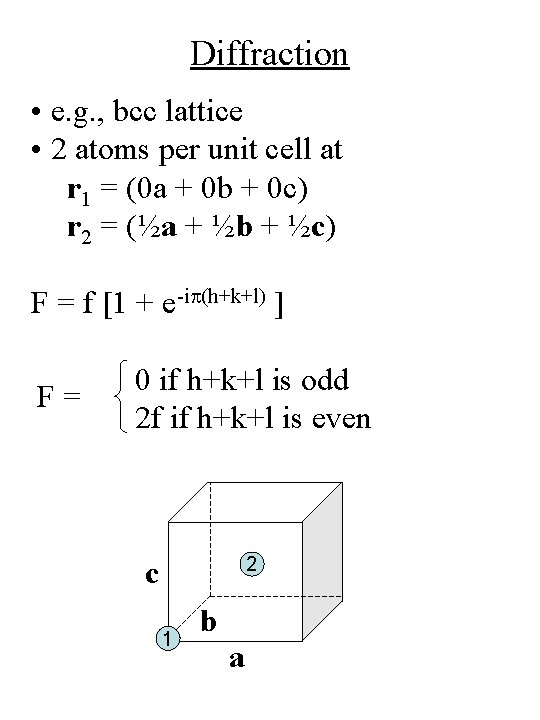
Diffraction • e. g. , bcc lattice • 2 atoms per unit cell at r 1 = (0 a + 0 b + 0 c) r 2 = (½a + ½b + ½c) F = f [1 + e-ip(h+k+l) ] F= 0 if h+k+l is odd 2 f if h+k+l is even 2 c 1 b a

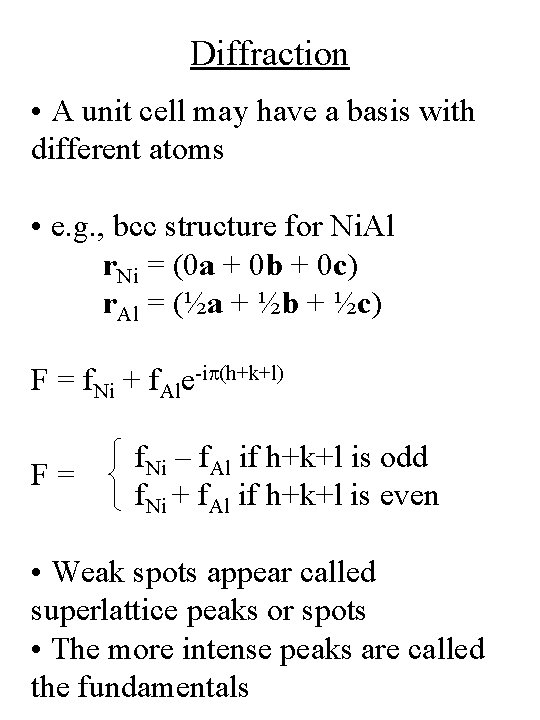
Diffraction • A unit cell may have a basis with different atoms • e. g. , bcc structure for Ni. Al r. Ni = (0 a + 0 b + 0 c) r. Al = (½a + ½b + ½c) F = f. Ni + f. Ale-ip(h+k+l) F= f. Ni – f. Al if h+k+l is odd f. Ni + f. Al if h+k+l is even • Weak spots appear called superlattice peaks or spots • The more intense peaks are called the fundamentals

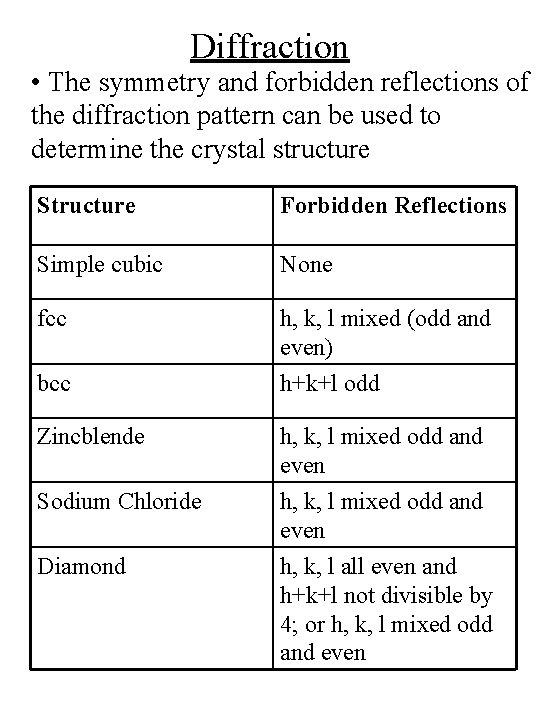
Diffraction • The symmetry and forbidden reflections of the diffraction pattern can be used to determine the crystal structure Structure Forbidden Reflections Simple cubic None fcc h, k, l mixed (odd and even) bcc h+k+l odd Zincblende h, k, l mixed odd and even h, k, l all even and h+k+l not divisible by 4; or h, k, l mixed odd and even Sodium Chloride Diamond

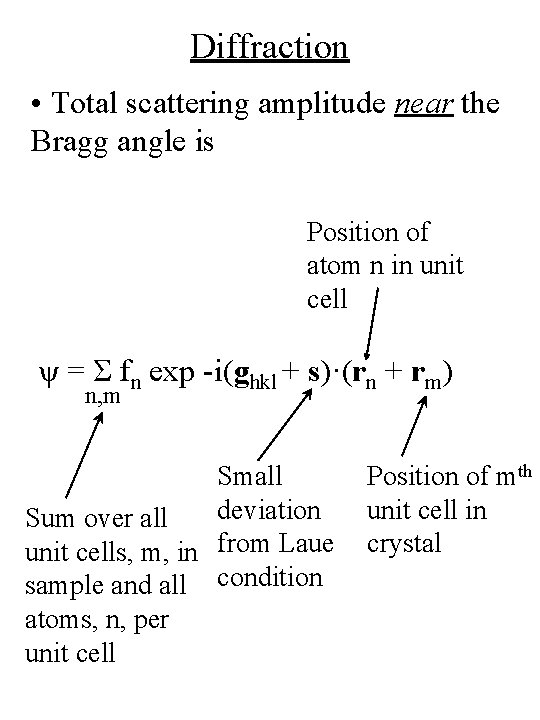
Diffraction • Total scattering amplitude near the Bragg angle is Position of atom n in unit cell ψ = Σ fn exp -i(ghkl + s)·(rn + rm) n, m Small deviation Sum over all unit cells, m, in from Laue sample and all condition atoms, n, per unit cell Position of mth unit cell in crystal

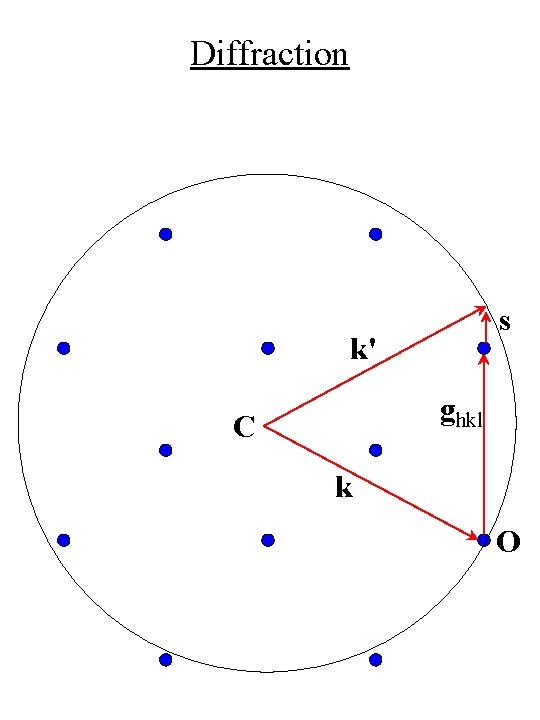
Diffraction s k' ghkl C k O

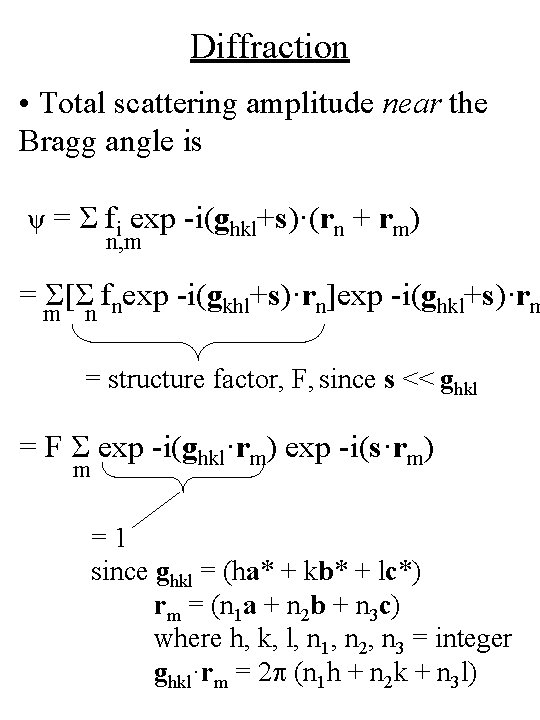
Diffraction • Total scattering amplitude near the Bragg angle is ψ = Σ fi exp -i(ghkl+s)·(rn + rm) n, m = Σ[Σ fnexp -i(gkhl+s)·rn]exp -i(ghkl+s)·rm m n = structure factor, F, since s << ghkl = F Σ exp -i(ghkl·rm) exp -i(s·rm) m =1 since ghkl = (ha* + kb* + lc*) rm = (n 1 a + n 2 b + n 3 c) where h, k, l, n 1, n 2, n 3 = integer ghkl·rm = 2 p (n 1 h + n 2 k + n 3 l)

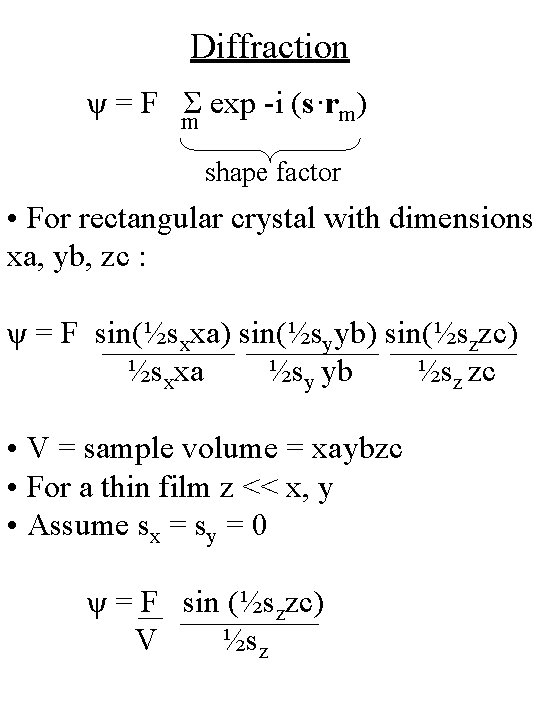
Diffraction ψ = F Σ exp -i (s·rm) m shape factor • For rectangular crystal with dimensions xa, yb, zc : ψ = F sin(½sxxa) sin(½syyb) sin(½szzc) ½sxxa ½sy yb ½sz zc • V = sample volume = xaybzc • For a thin film z << x, y • Assume sx = sy = 0 ψ = F sin (½szzc) V ½sz

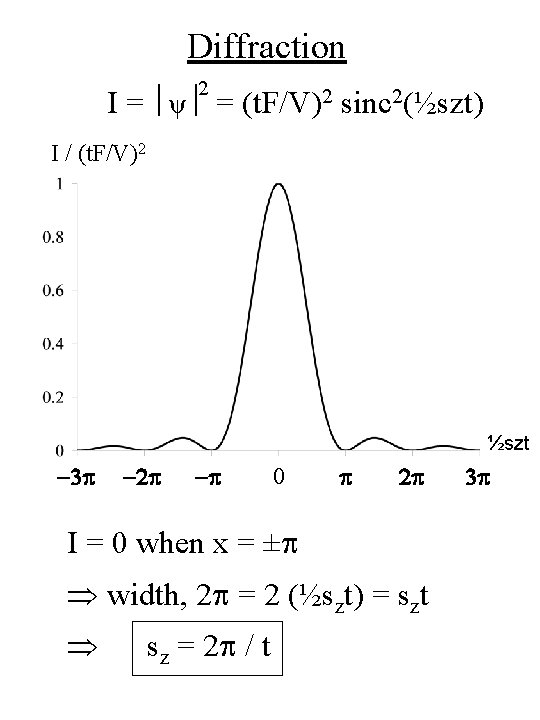
Diffraction ψ = F sin (½szzc) V ½sz • Now zc = film thickness, t ψ = F sin (½szt) V ½sz • Intensity of diffracted wave is : 2 I = ψ = (F/V)2 [( sin(½szt) )/(½sz)]2 = (t. F/V)2 sinc 2(½szt)

Diffraction 2 I = ψ = (t. F/V)2 sinc 2(½szt) I / (t. F/V)2 ½szt -3 p -2 p -p 0 p 2 p I = 0 when x = ±p width, 2 p = 2 (½szt) = szt sz = 2 p / t 3 p

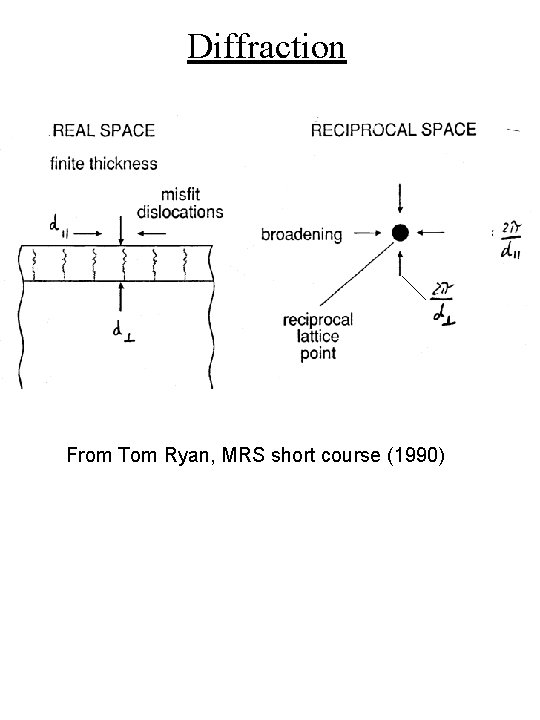
Diffraction • Due to finite thickness of the film, each reciprocal lattice point may be considered as streaks with length 2 p/t k' ghkl C k O

Diffraction • The thinner the film the greater the “streaking” • Lattice points become lattice rods for very thin films (monolayers) from Ohring, Fig. 7 -27, p. 346

Diffraction From Tom Ryan, MRS short course (1990)