Thermodynamics ME 1229 Credit 3 0 Energy Transfer




- Slides: 32

Thermodynamics ME 1229 Credit: 3. 0 Energy Transfer (Heat & Work) Presented By Md. Shariful Islam Lecturer Department of Mechanical Engineering Khulna University of Engineering & Technology

q. Energy is the capacity to exert a force through a distance & manifest itself in various forms e. g. mechanical, electric, nuclear, chemical. q. Engineering processes involves the conversion of energy from one form to another. Energy 2

q. There are 2 types of energy q. Stored energy q. Energy in transition Energy q. Stored Energy: It is the type of energy which is contained within the system boundary. Example. Potential energy, Kinetic energy q. Energy in Transition: It is the energy which crosses the system boundary. Example. Heat, work 3

q. Energy can be transferred to or from a closed system (a fixed mass) in two distinct forms: Energy Heat and Work q. An energy transfer to or from a closed system is heat if it is caused by a temperature difference. q. Otherwise it is work, and it is caused by a force acting through a distance. 4

Heat q. Heat is the interaction between system and surroundings which occurs by virtue of their temperature difference. q. Heat always flows from hot body to cold body naturally. q. External work should be done to transfer heat from cold body to hot body. q. The unit of heat is k. J 5

Energy transfer by work (work transfer) 6

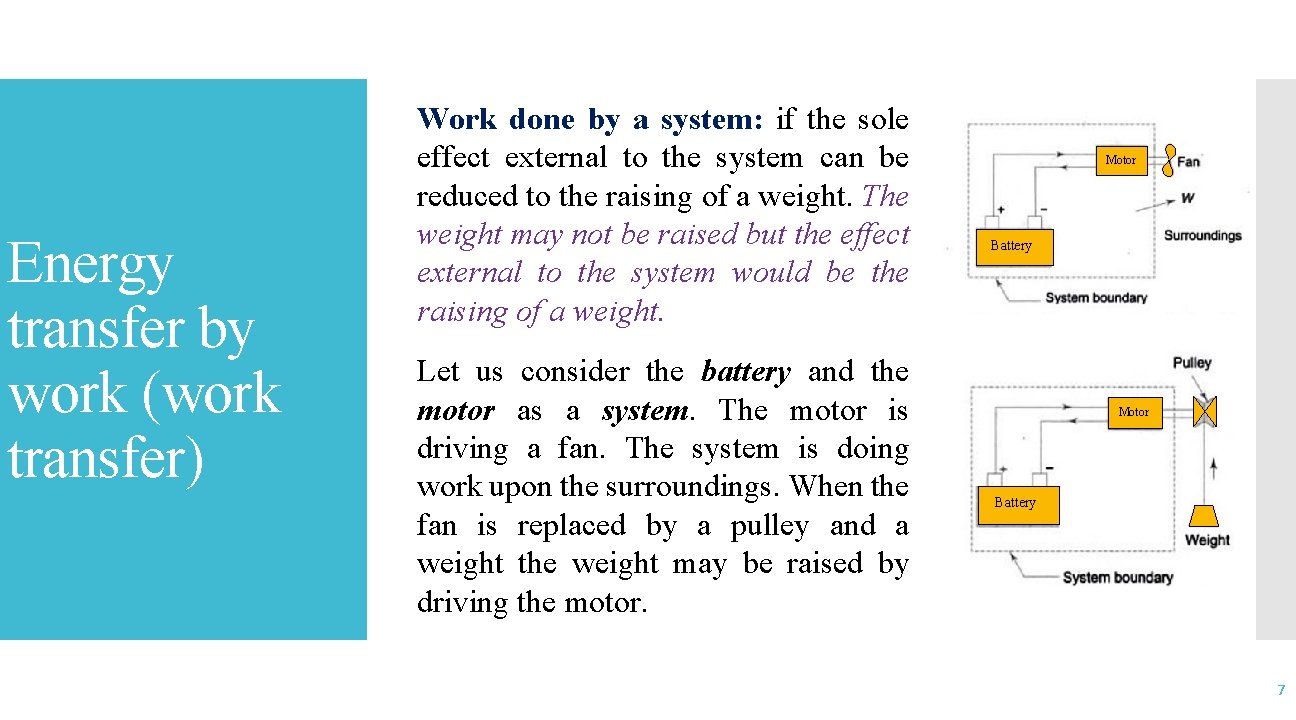
Energy transfer by work (work transfer) Work done by a system: if the sole effect external to the system can be reduced to the raising of a weight. The weight may not be raised but the effect external to the system would be the raising of a weight. Let us consider the battery and the motor as a system. The motor is driving a fan. The system is doing work upon the surroundings. When the fan is replaced by a pulley and a weight the weight may be raised by driving the motor. Motor Battery 7

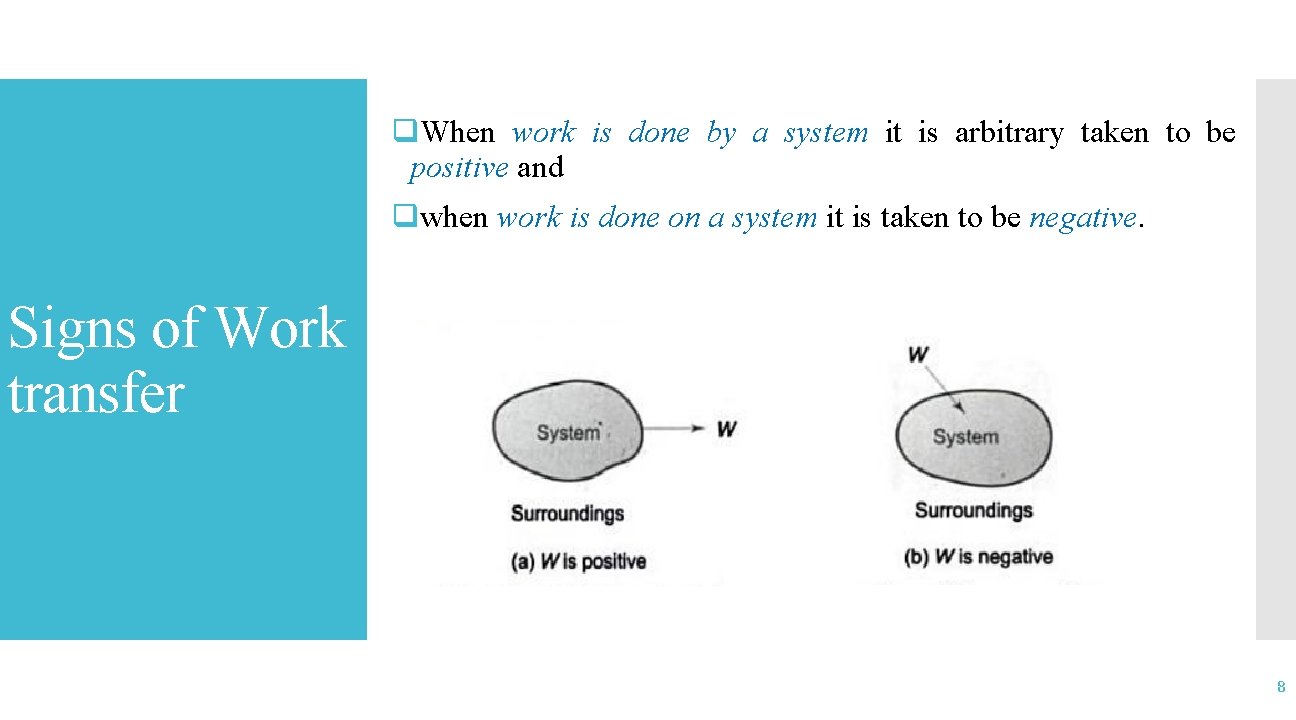
q. When work is done by a system it is arbitrary taken to be positive and qwhen work is done on a system it is taken to be negative. Signs of Work transfer 8

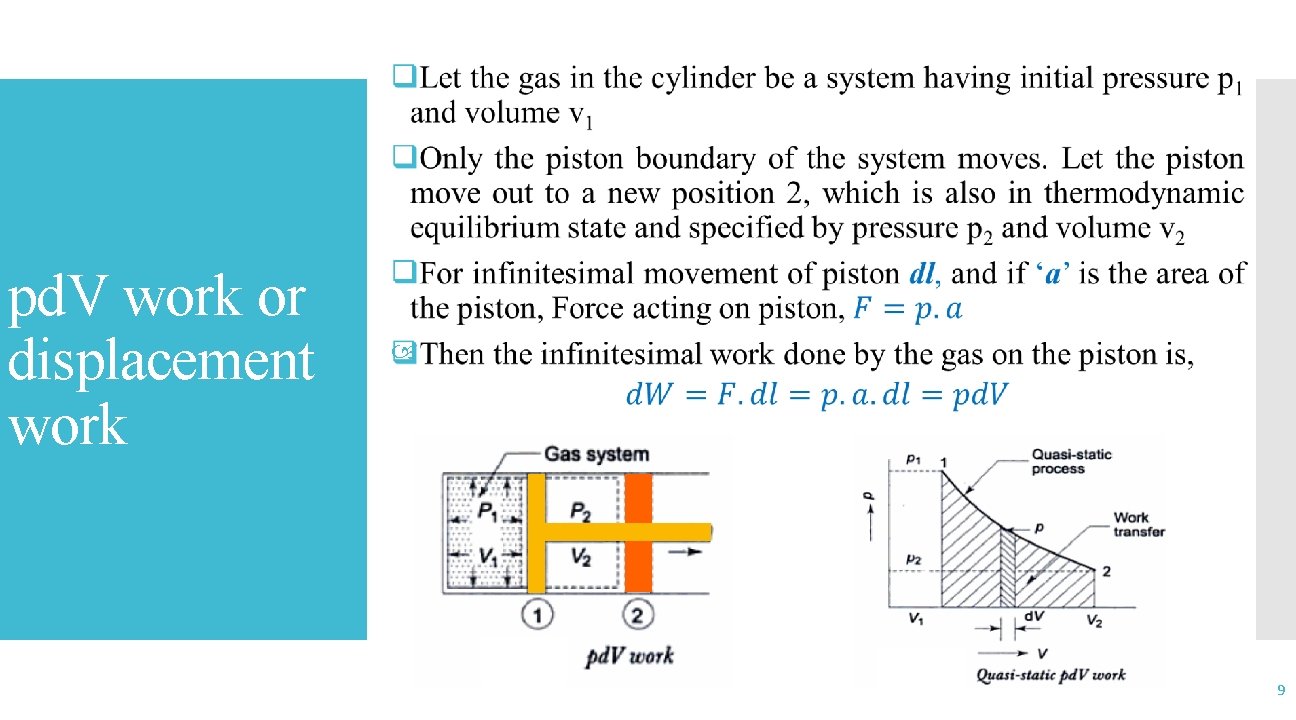
pd. V work or displacement work 9

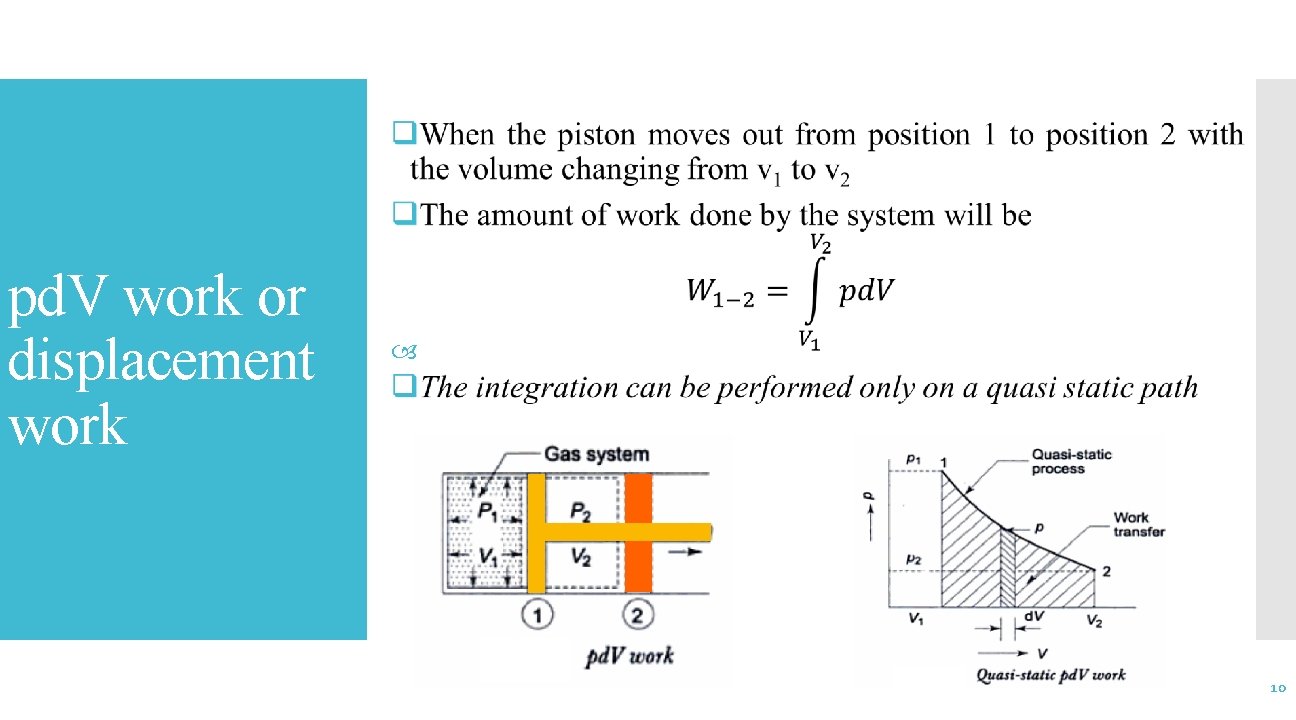
pd. V work or displacement work 10

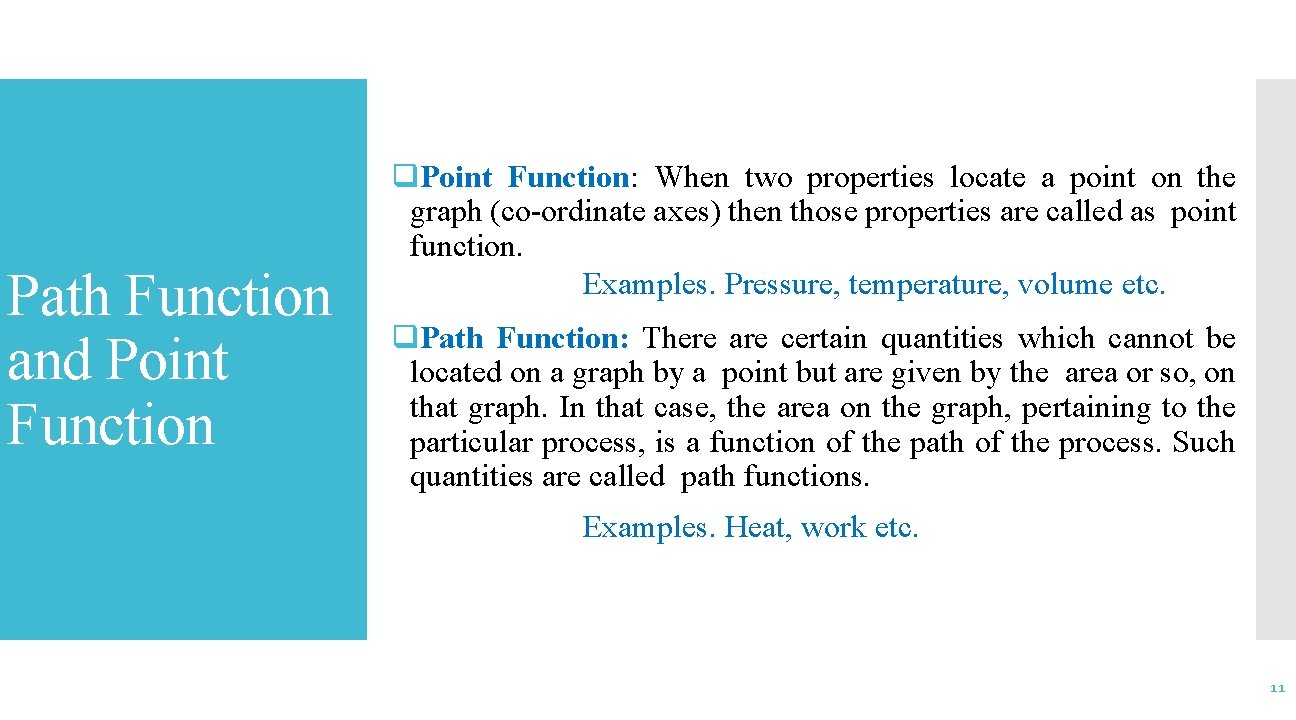
Path Function and Point Function q. Point Function: When two properties locate a point on the graph (co-ordinate axes) then those properties are called as point function. Examples. Pressure, temperature, volume etc. q. Path Function: There are certain quantities which cannot be located on a graph by a point but are given by the area or so, on that graph. In that case, the area on the graph, pertaining to the particular process, is a function of the path of the process. Such quantities are called path functions. Examples. Heat, work etc. 11

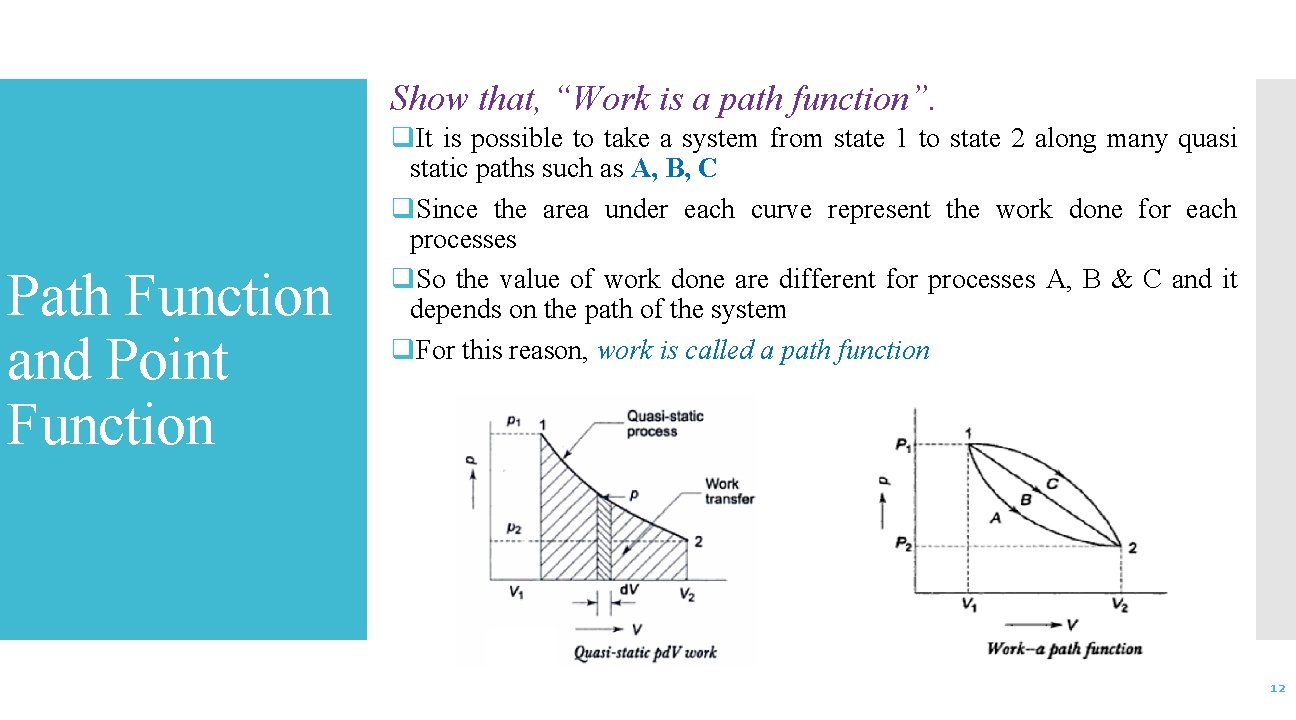
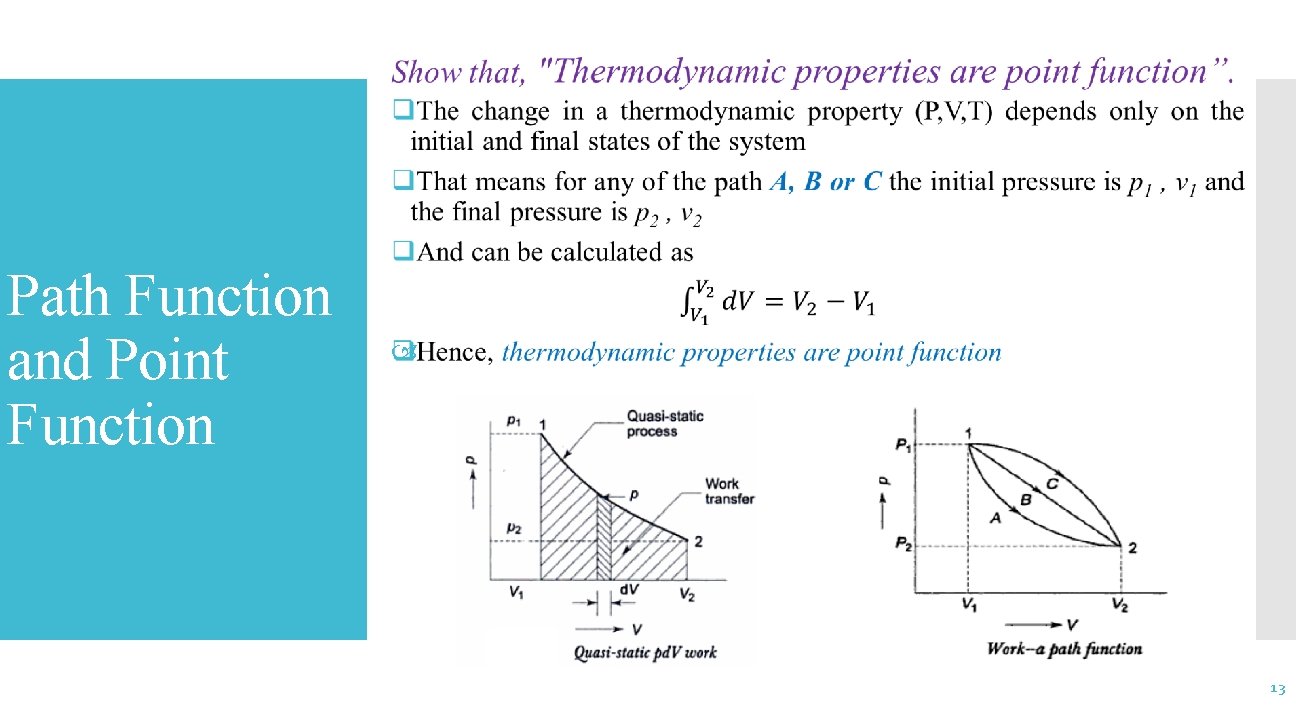
Show that, “Work is a path function”. Path Function and Point Function q. It is possible to take a system from state 1 to state 2 along many quasi static paths such as A, B, C q. Since the area under each curve represent the work done for each processes q. So the value of work done are different for processes A, B & C and it depends on the path of the system q. For this reason, work is called a path function 12

Path Function and Point Function 13

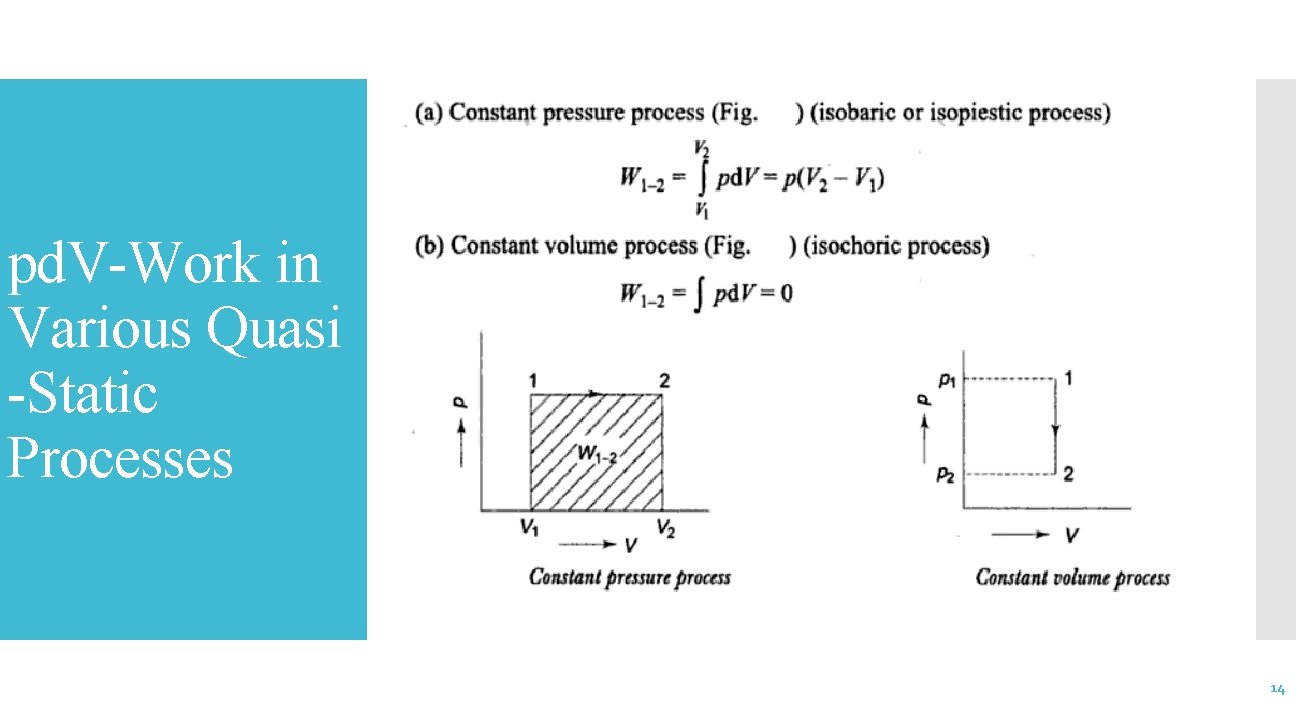
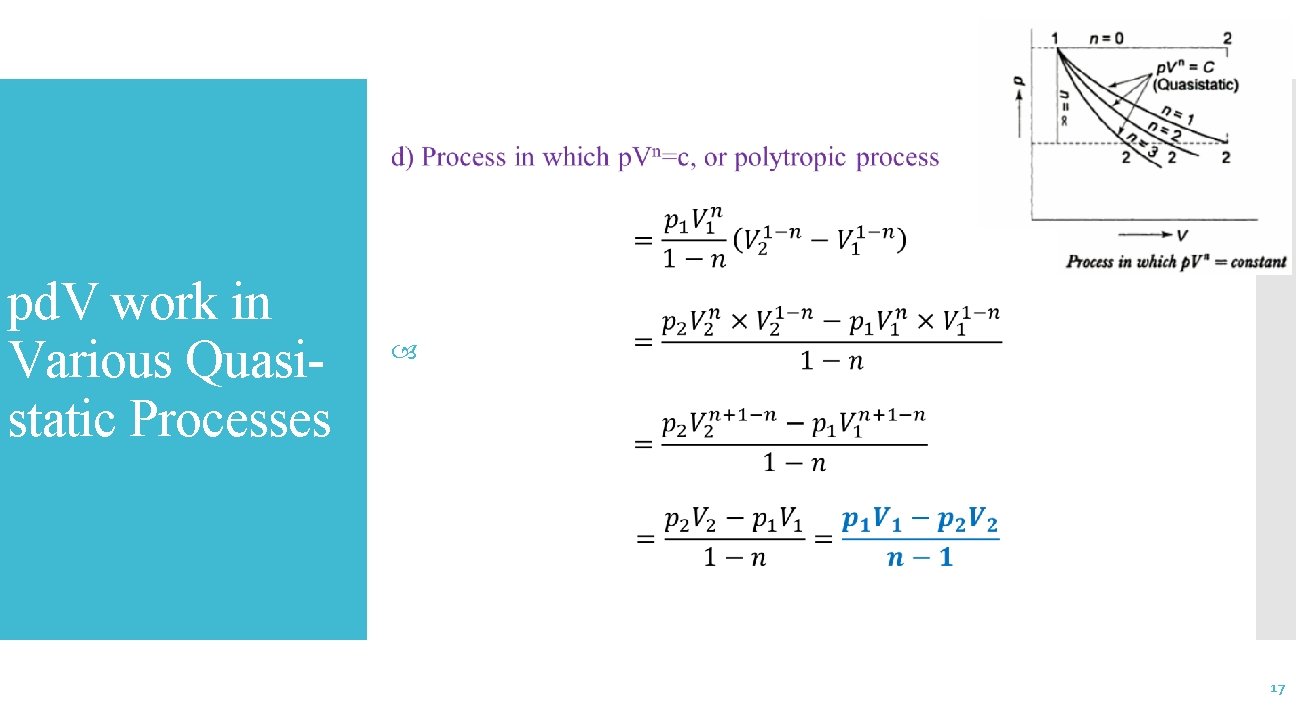
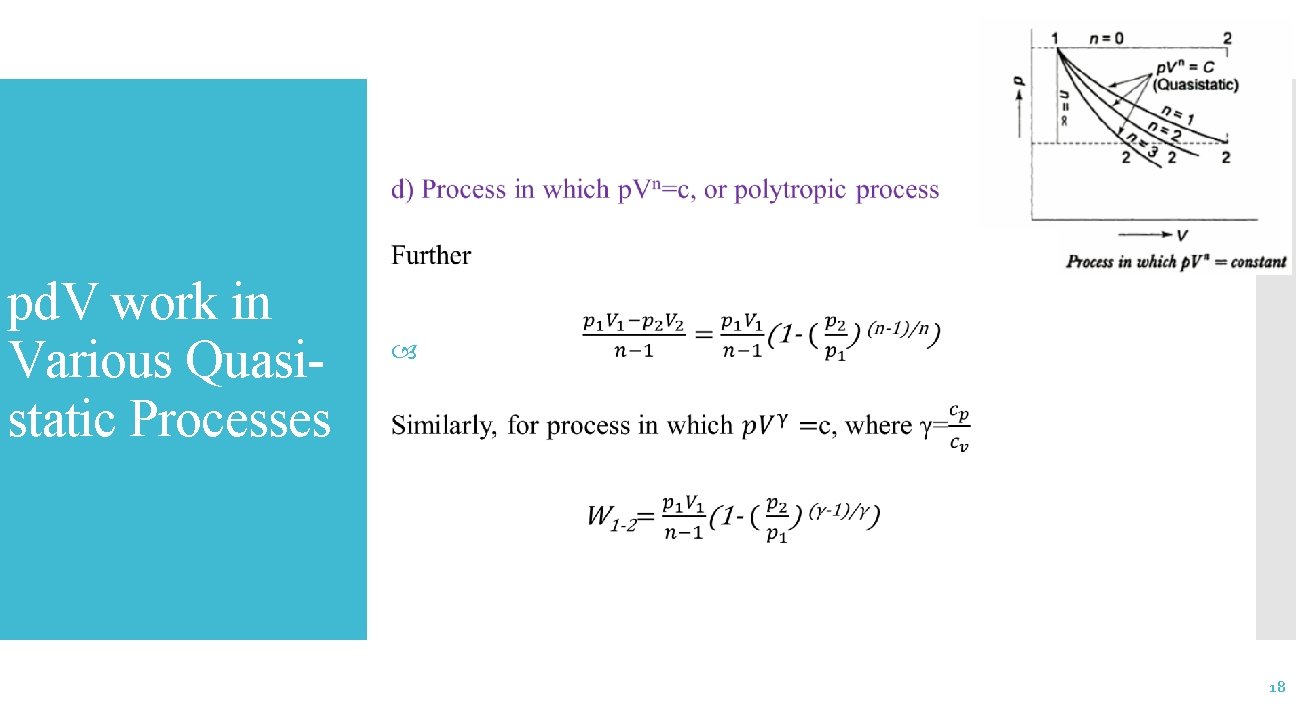
pd. V-Work in Various Quasi -Static Processes 14

pd. V work in Various Quasistatic Processes 15

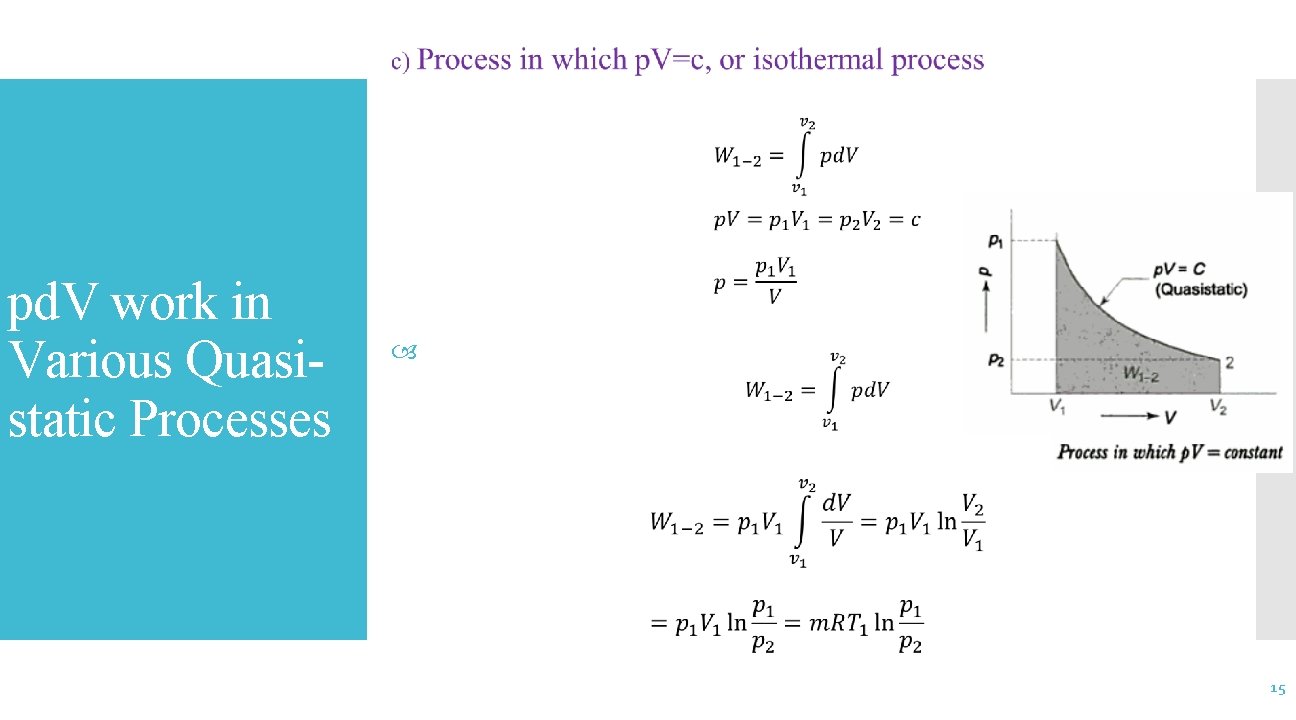
pd. V work in Various Quasistatic Processes 16

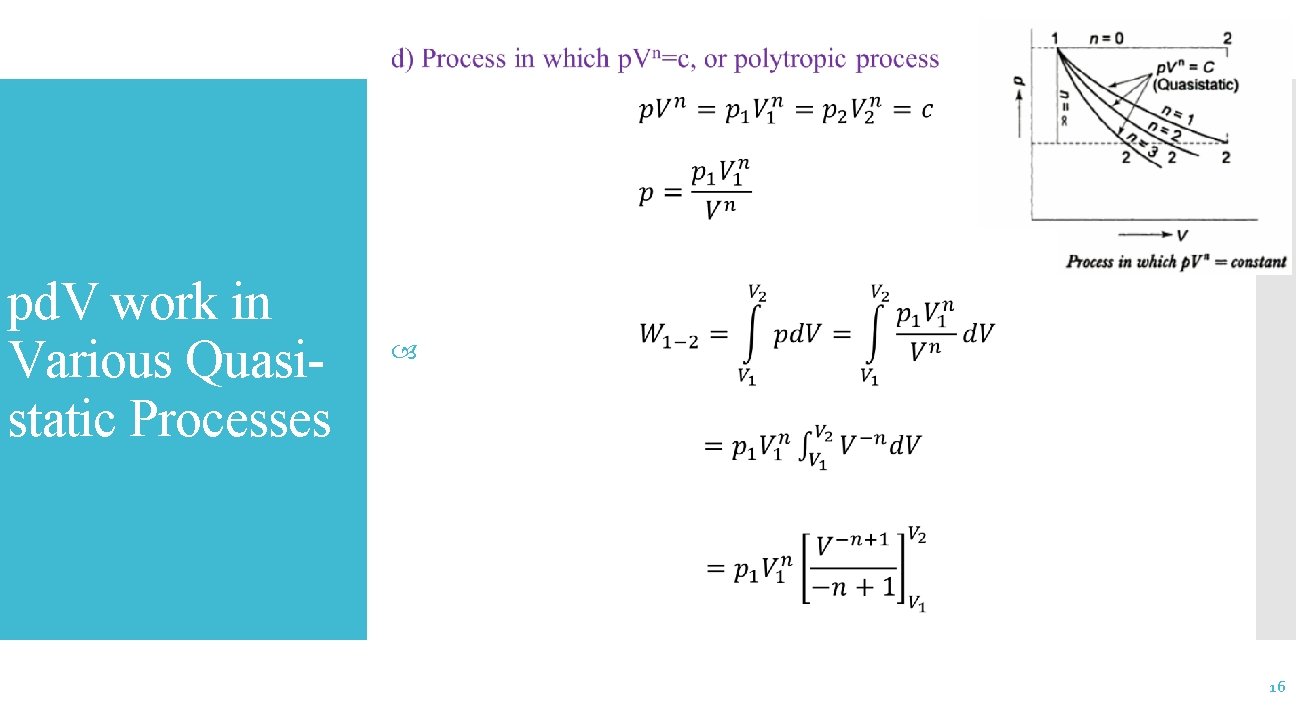
pd. V work in Various Quasistatic Processes 17

pd. V work in Various Quasistatic Processes 18

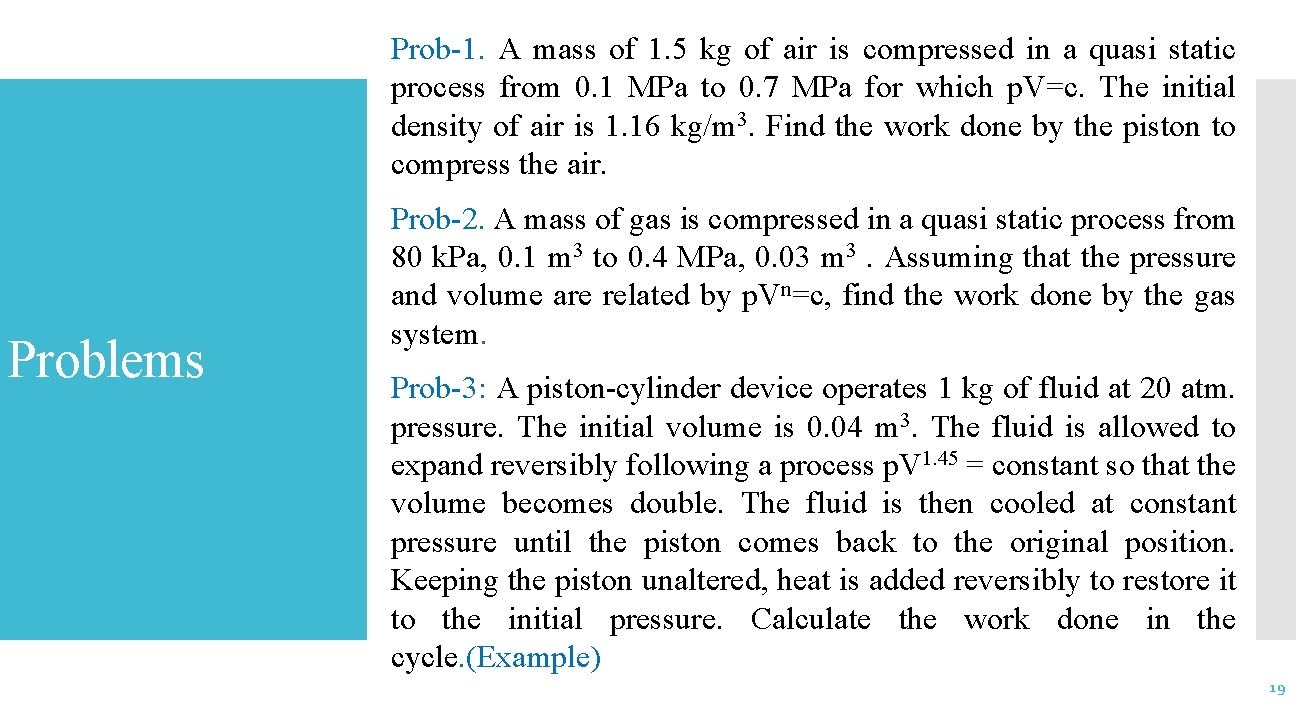
Prob-1. A mass of 1. 5 kg of air is compressed in a quasi static process from 0. 1 MPa to 0. 7 MPa for which p. V=c. The initial density of air is 1. 16 kg/m 3. Find the work done by the piston to compress the air. Problems Prob-2. A mass of gas is compressed in a quasi static process from 80 k. Pa, 0. 1 m 3 to 0. 4 MPa, 0. 03 m 3. Assuming that the pressure and volume are related by p. Vn=c, find the work done by the gas system. Prob-3: A piston-cylinder device operates 1 kg of fluid at 20 atm. pressure. The initial volume is 0. 04 m 3. The fluid is allowed to expand reversibly following a process p. V 1. 45 = constant so that the volume becomes double. The fluid is then cooled at constant pressure until the piston comes back to the original position. Keeping the piston unaltered, heat is added reversibly to restore it to the initial pressure. Calculate the work done in the cycle. (Example) 19

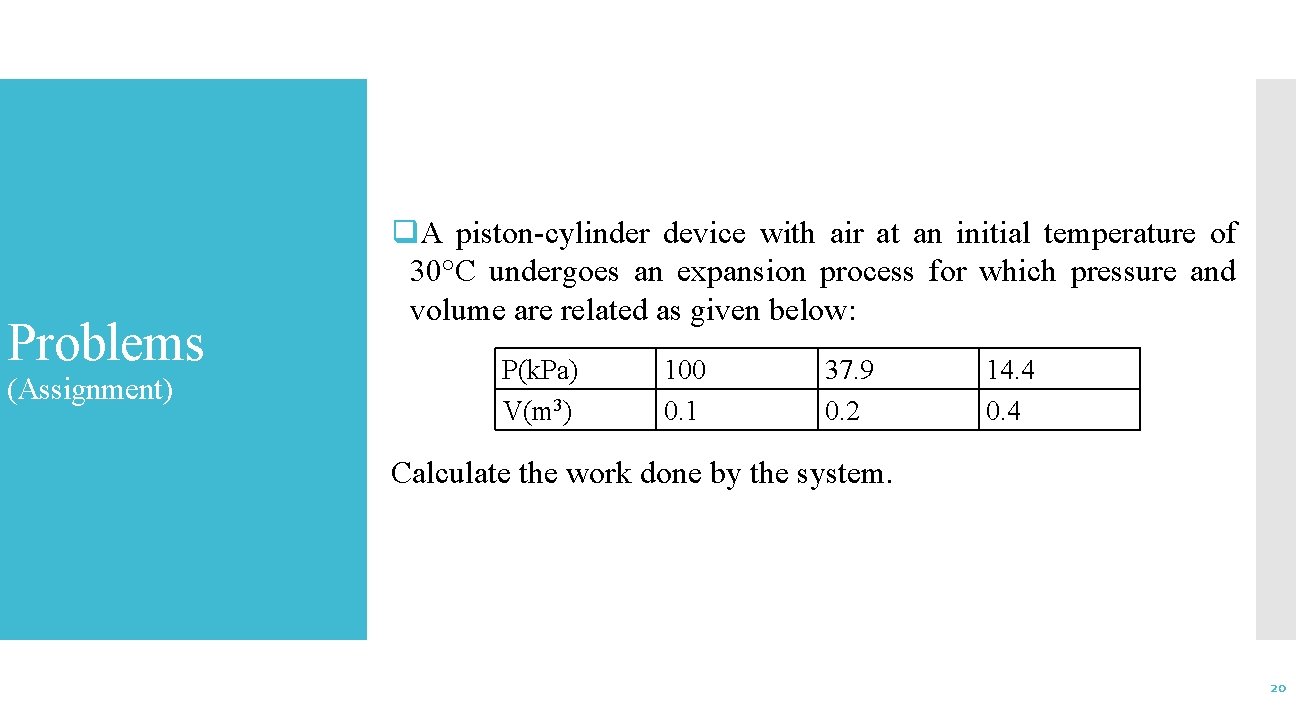
Problems (Assignment) q. A piston-cylinder device with air at an initial temperature of 30°C undergoes an expansion process for which pressure and volume are related as given below: P(k. Pa) V(m 3) 100 0. 1 37. 9 0. 2 14. 4 0. 4 Calculate the work done by the system. 20

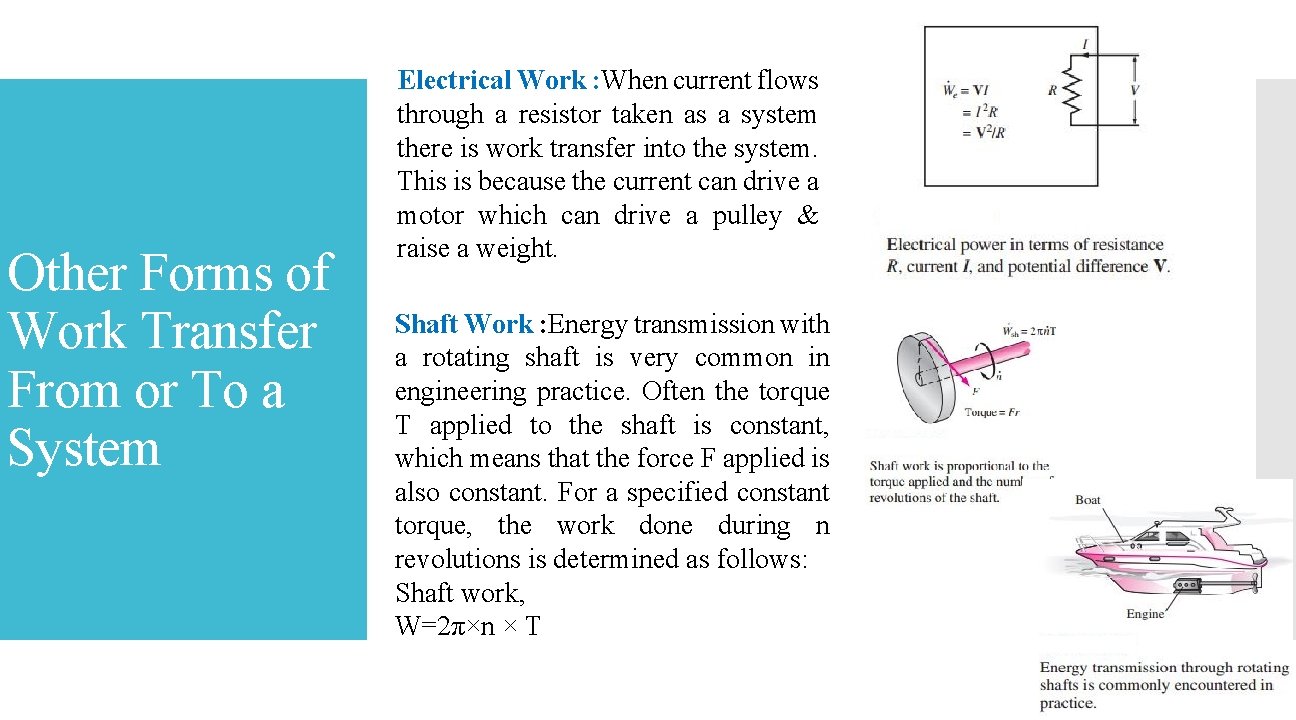
Other Forms of Work Transfer From or To a System Electrical Work : When current flows through a resistor taken as a system there is work transfer into the system. This is because the current can drive a motor which can drive a pulley & raise a weight. Shaft Work : Energy transmission with a rotating shaft is very common in engineering practice. Often the torque T applied to the shaft is constant, which means that the force F applied is also constant. For a specified constant torque, the work done during n revolutions is determined as follows: Shaft work, W=2π×n × T 21

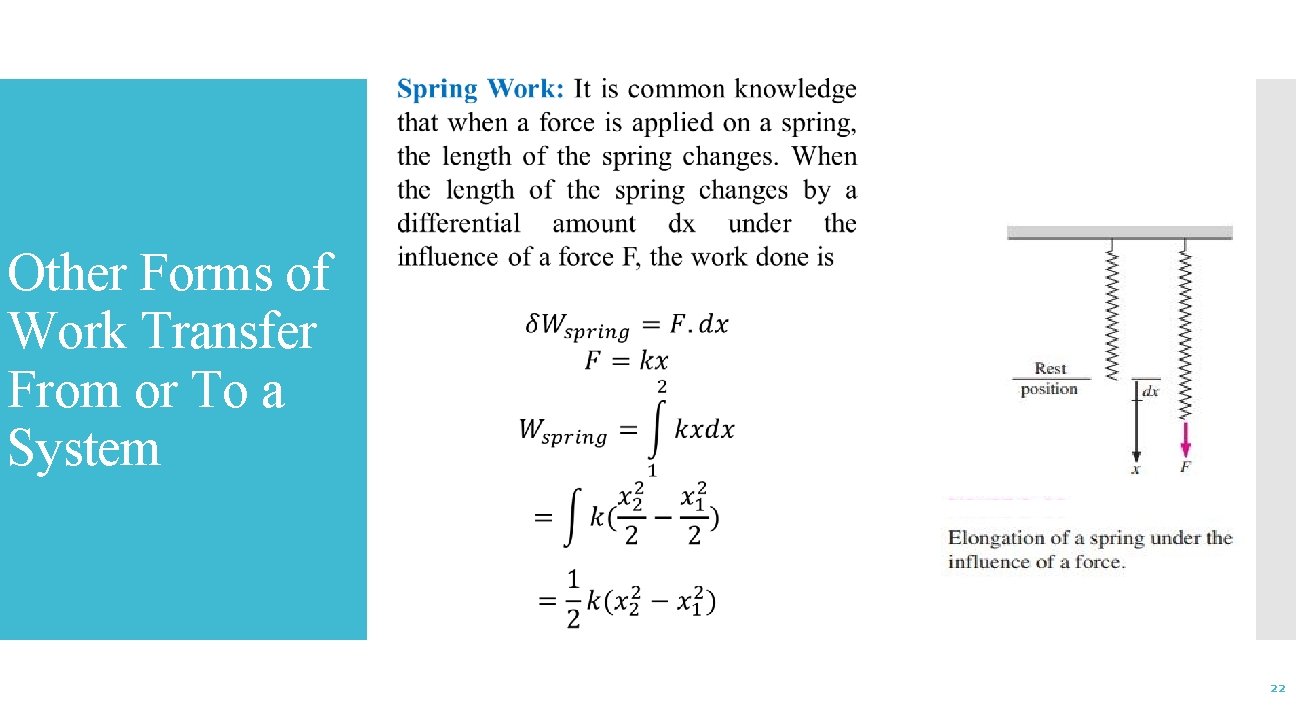
Other Forms of Work Transfer From or To a System 22

Net Work Done By a System 23

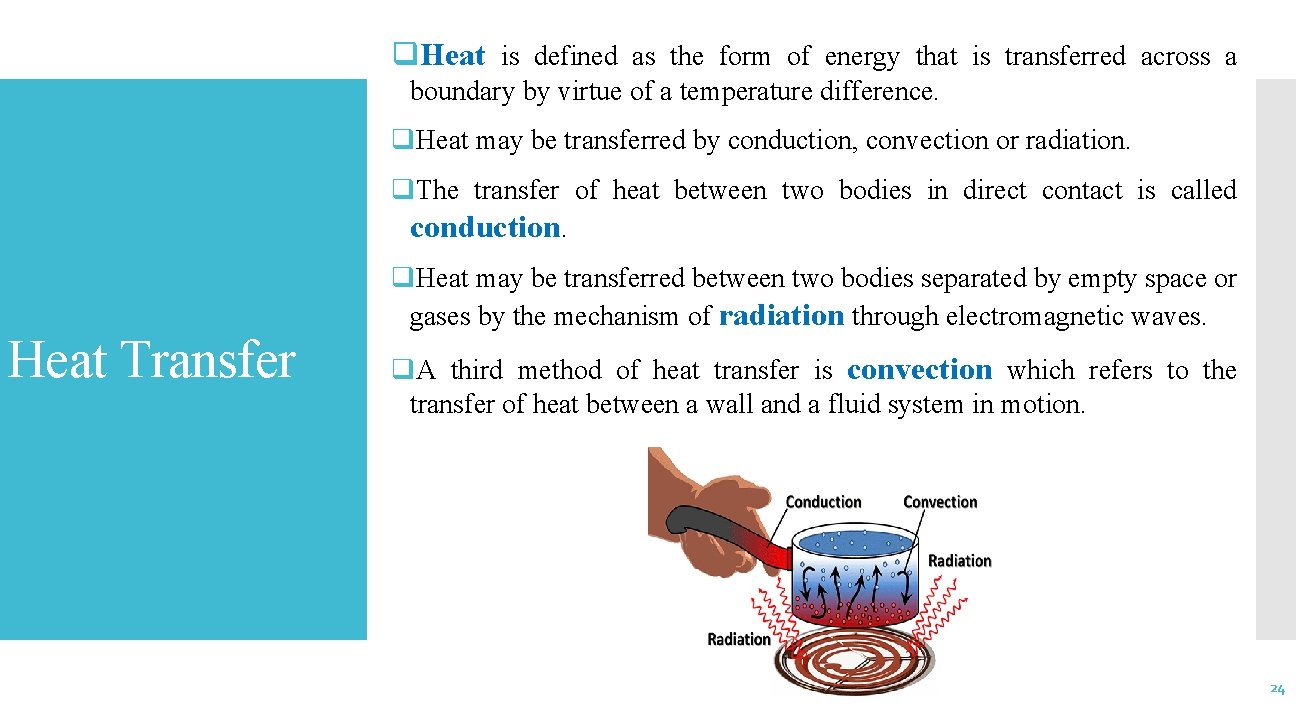
q. Heat is defined as the form of energy that is transferred across a boundary by virtue of a temperature difference. q. Heat may be transferred by conduction, convection or radiation. q. The transfer of heat between two bodies in direct contact is called conduction. Heat Transfer q. Heat may be transferred between two bodies separated by empty space or gases by the mechanism of radiation through electromagnetic waves. q. A third method of heat transfer is convection which refers to the transfer of heat between a wall and a fluid system in motion. 24

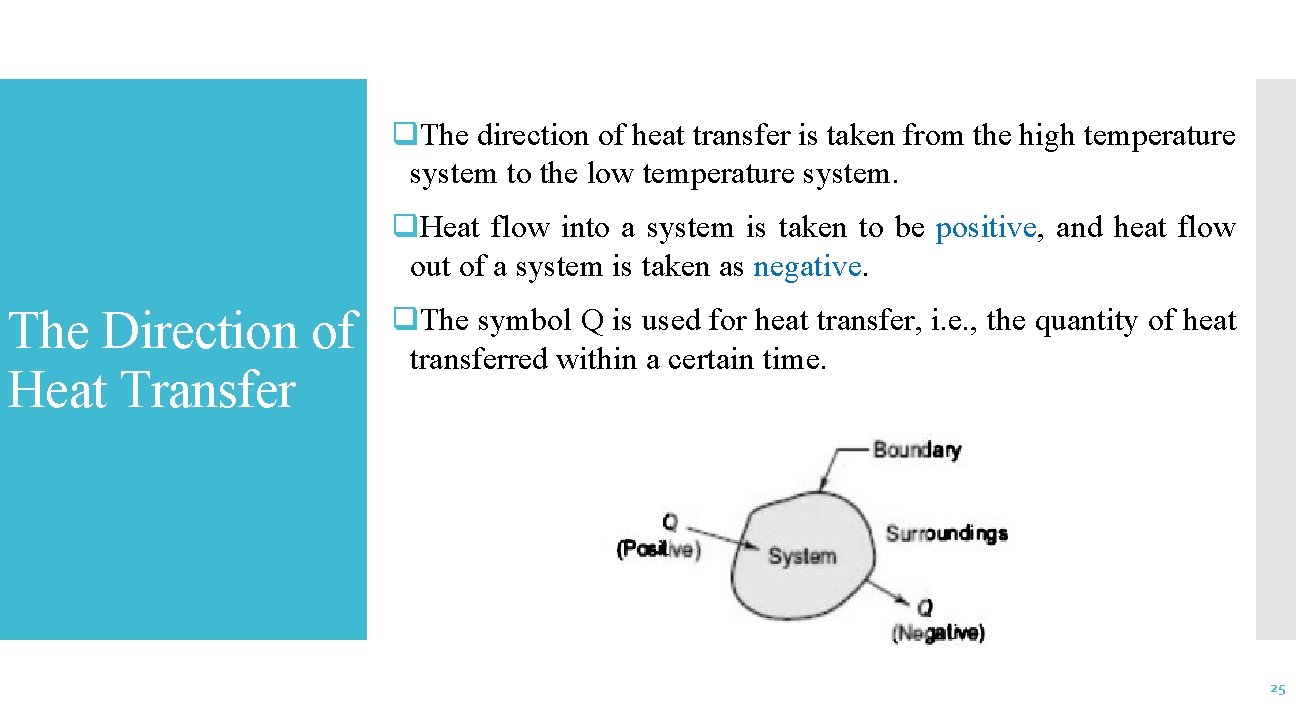
q. The direction of heat transfer is taken from the high temperature system to the low temperature system. q. Heat flow into a system is taken to be positive, and heat flow out of a system is taken as negative. The Direction of Heat Transfer q. The symbol Q is used for heat transfer, i. e. , the quantity of heat transferred within a certain time. 25

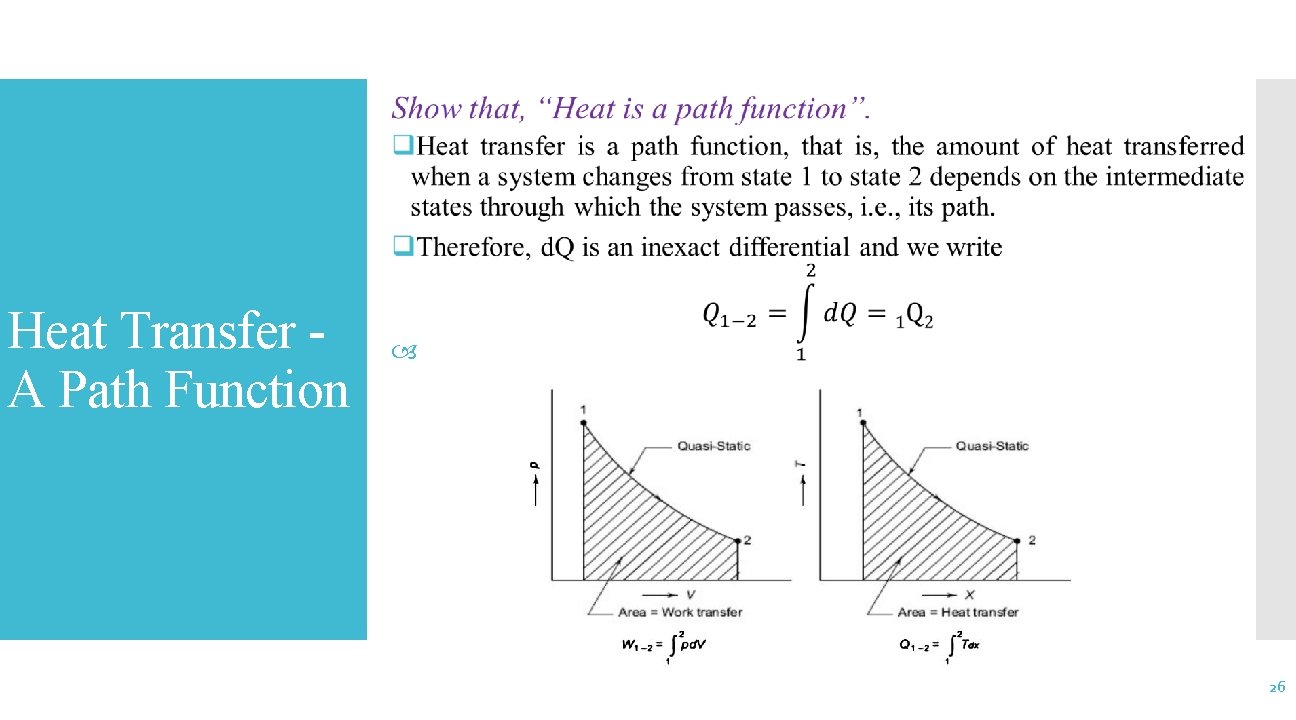
Heat Transfer A Path Function 26

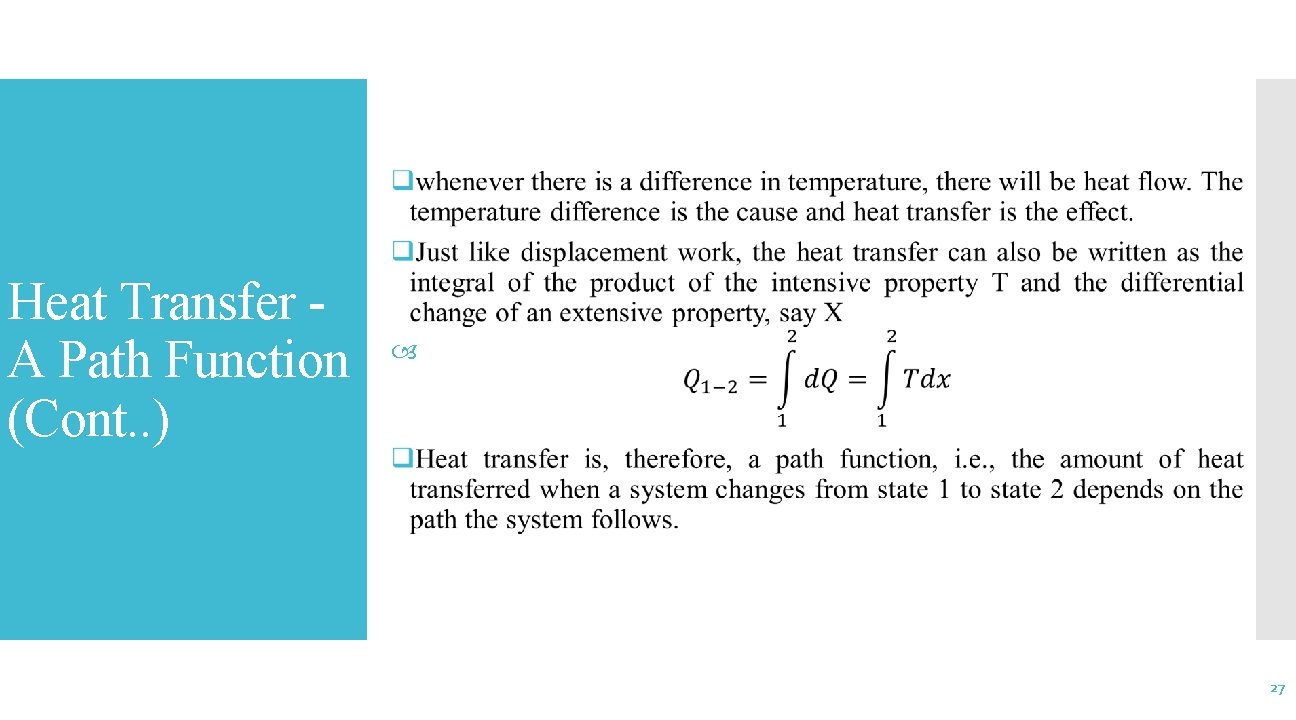
Heat Transfer A Path Function (Cont. . ) 27

q“A process during which there is no heat transfer is called an adiabatic process. ” q. The word adiabatic comes from the Greek word adiabatos, which means not to be passed. q. There are 2 ways a process can be adiabatic. Adiabatic Process qthe system is well insulated so that only a negligible amount of heat can pass through the boundary. qboth the system and the surroundings are at the same temperature and therefore there is no driving force (temperature difference) for heat transfer. 28

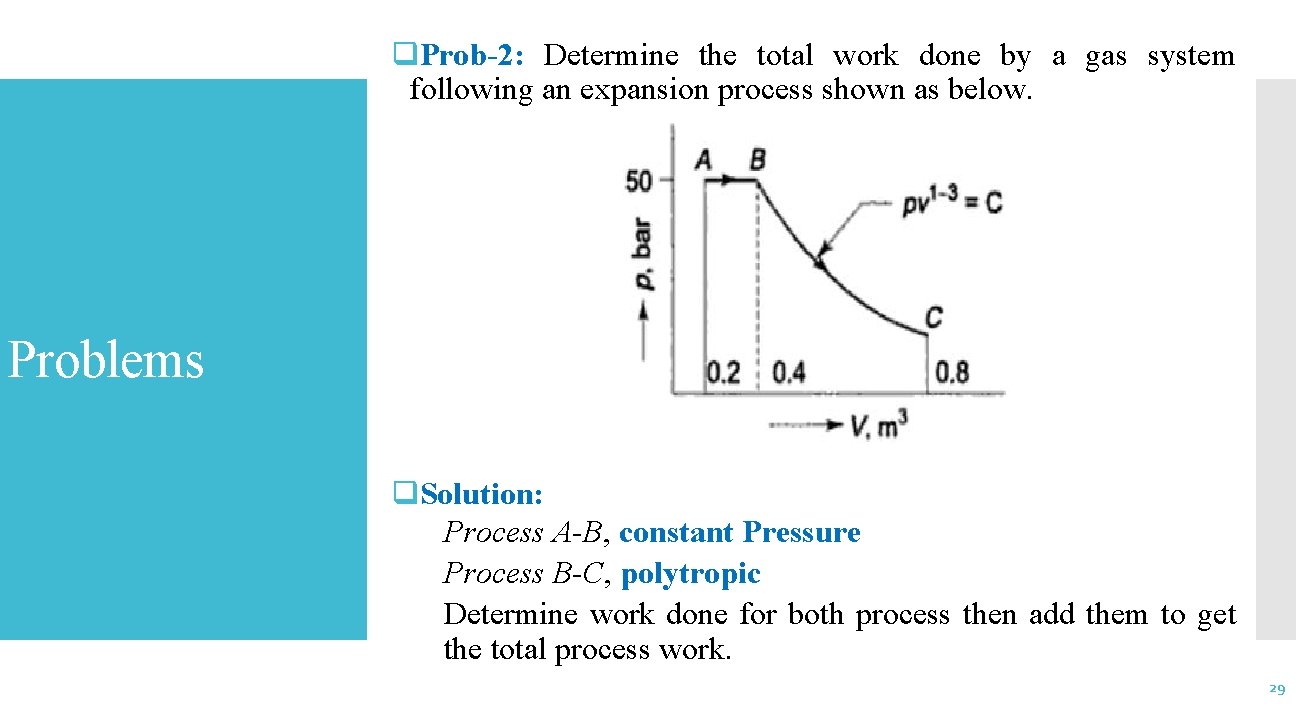
q. Prob-2: Determine the total work done by a gas system following an expansion process shown as below. Problems q. Solution: Process A-B, constant Pressure Process B-C, polytropic Determine work done for both process then add them to get the total process work. 29

Problems q. Prob-1: A fluid at 4. 15 bar is expanded reversibly according to a law p. V = constant to a pressure of 1. 15 bar until it has a specific volume of 0. 12 m 3/kg. It is then cooled reversibly at a constant pressure, then is cooled at constant volume until the pressure is 0. 62 bar; and is then allowed to compress reversibly according to a law p. Vn = constant back to the initial conditions. The work done in the constant pressure is 0. 525 k. J, and the mass of fluid present is 0. 22 kg. Calculate the value of n in the fourth process, the net work of the cycle and sketch the cycle on a p-V diagram. 30

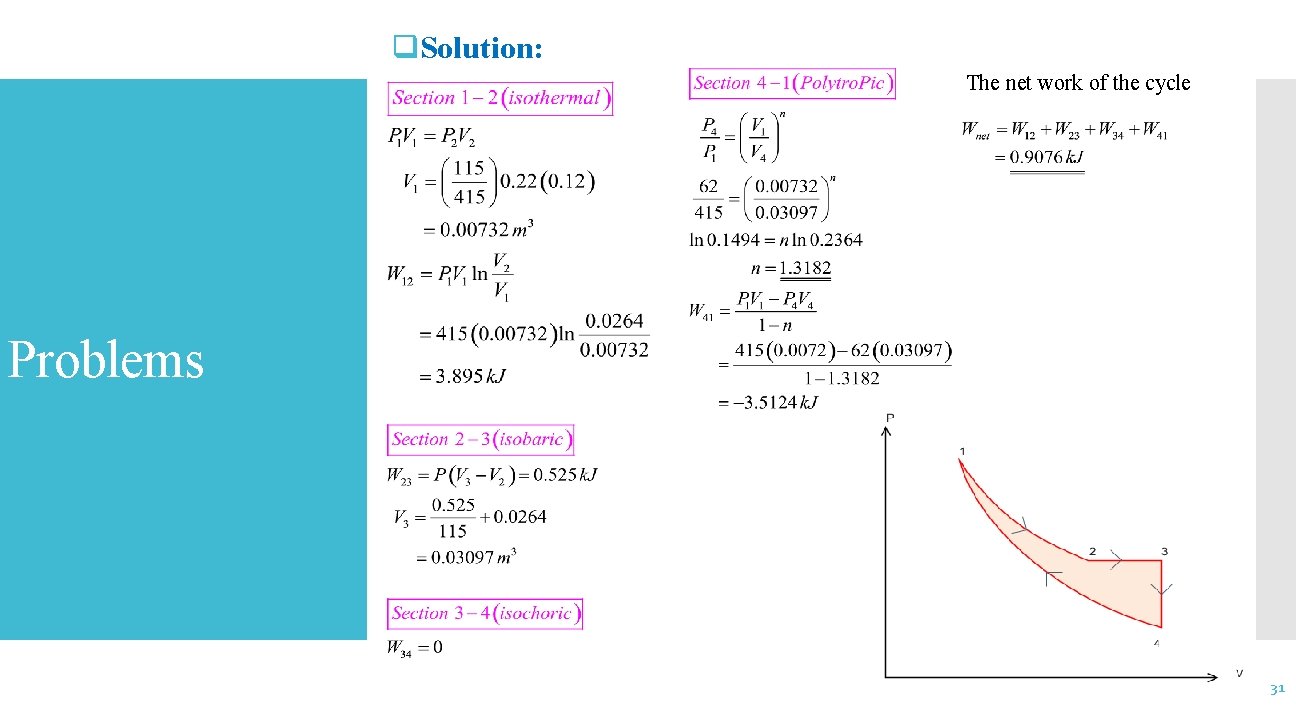
q. Solution: The net work of the cycle Problems 31

Thank You 9/9/2021 Md. Shariful Islam, Lecturer, Department of Mechanical Engineering, KUET 32
 Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis This can be avoided by giving credit where credit is due.
This can be avoided by giving credit where credit is due. Unc transfer credit
Unc transfer credit Umd transfer credit
Umd transfer credit European credit transfer and accumulation system
European credit transfer and accumulation system Internal energy formula thermodynamics
Internal energy formula thermodynamics Sssf thermodynamics
Sssf thermodynamics Internal energy in thermodynamics definition
Internal energy in thermodynamics definition Laws in thermodynamics
Laws in thermodynamics Conservation of energy thermodynamics
Conservation of energy thermodynamics Internal energy formula thermodynamics
Internal energy formula thermodynamics Thermal.energy formula
Thermal.energy formula A wave is a disturbance that transfers
A wave is a disturbance that transfers Work is the transfer of energy
Work is the transfer of energy Is work a transfer of energy
Is work a transfer of energy What is a repeating disturbance
What is a repeating disturbance Waves transfer energy without transferring
Waves transfer energy without transferring Roles in energy transfer
Roles in energy transfer Difference between conduction convection and radiation
Difference between conduction convection and radiation Ancient egypt pyramid of power
Ancient egypt pyramid of power Examples of energy transfer
Examples of energy transfer Physics of archery
Physics of archery Flashlight energy transfer
Flashlight energy transfer Radiation heat transfer examples
Radiation heat transfer examples Rate of energy transfer by sinusoidal waves on strings
Rate of energy transfer by sinusoidal waves on strings Energy transfer in ecosystems
Energy transfer in ecosystems Energy storage and transfer model test answer key
Energy storage and transfer model test answer key Food webs and energy transfer
Food webs and energy transfer Energy transfer example
Energy transfer example Energy move
Energy move Energy flow diagram light bulb
Energy flow diagram light bulb Energy transferred equation
Energy transferred equation