Thermodiffusion in Polymer Solutions Jutta LuettmerStrathmann Department of



- Slides: 26

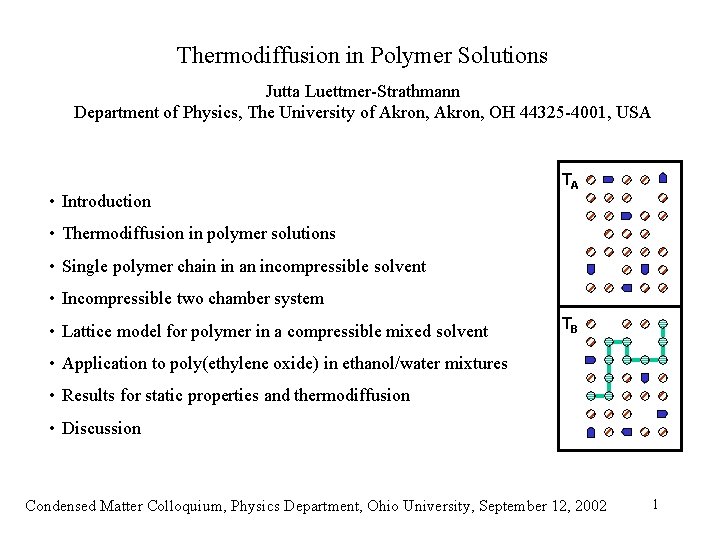
Thermodiffusion in Polymer Solutions Jutta Luettmer-Strathmann Department of Physics, The University of Akron, OH 44325 -4001, USA • Introduction TA • Thermodiffusion in polymer solutions • Single polymer chain in an incompressible solvent • Incompressible two chamber system • Lattice model for polymer in a compressible mixed solvent TB • Application to poly(ethylene oxide) in ethanol/water mixtures • Results for static properties and thermodiffusion • Discussion Condensed Matter Colloquium, Physics Department, Ohio University, September 12, 2002 1

Thanks to Mike Boiwka for performing Monte Carlo simulations 2

Thanks to Simone Wiegand, Berend Jan de Gans, and Rio Kita from the Max Planck Institut für Polymerforschung in Mainz for sharing their experimental data. 3

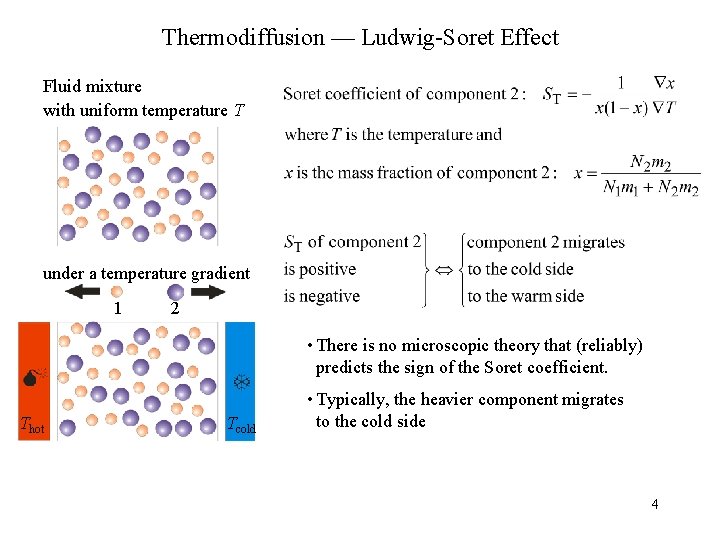
Thermodiffusion — Ludwig-Soret Effect Fluid mixture with uniform temperature T under a temperature gradient 1 2 • There is no microscopic theory that (reliably) predicts the sign of the Soret coefficient. Thot Tcold • Typically, the heavier component migrates to the cold side 4

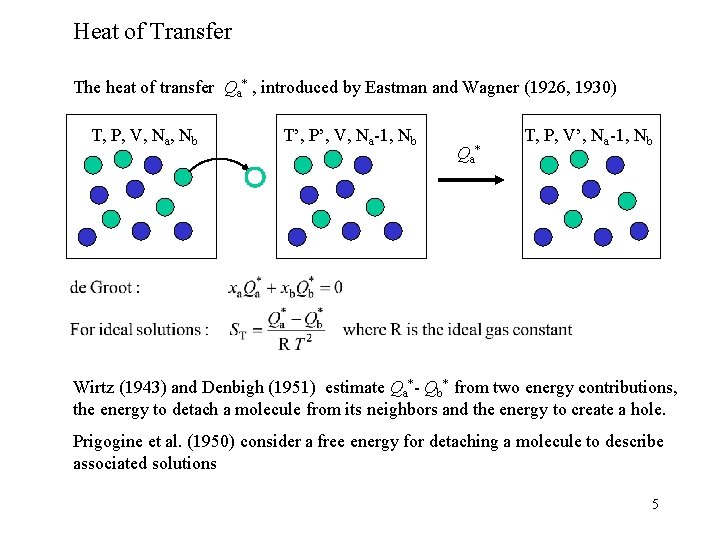
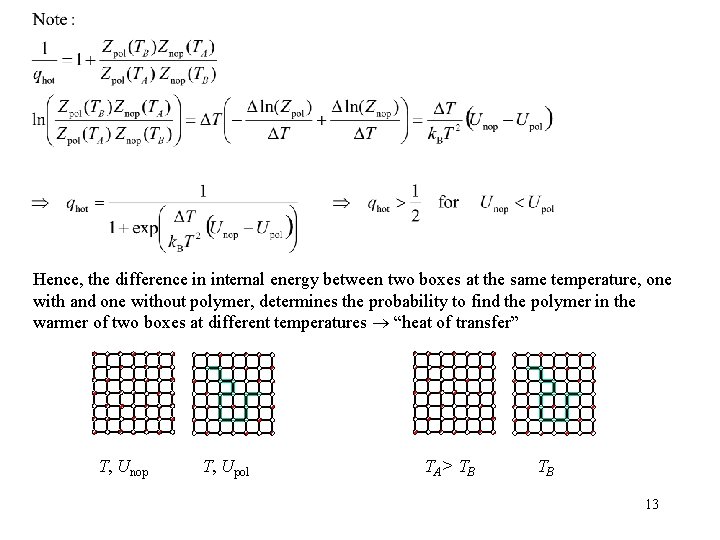
Heat of Transfer The heat of transfer Qa* , introduced by Eastman and Wagner (1926, 1930) T, P, V, Na, Nb T’, P’, V, Na-1, Nb Qa* T, P, V’, Na-1, Nb Wirtz (1943) and Denbigh (1951) estimate Qa*- Qb* from two energy contributions, the energy to detach a molecule from its neighbors and the energy to create a hole. Prigogine et al. (1950) consider a free energy for detaching a molecule to describe associated solutions 5

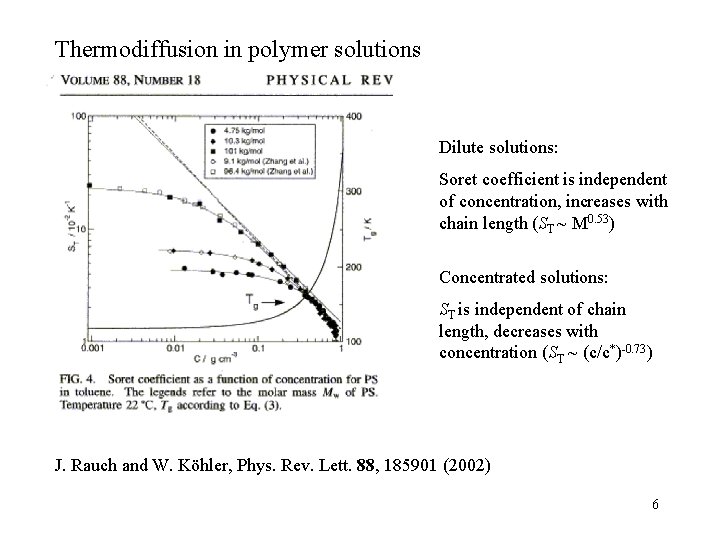
Thermodiffusion in polymer solutions Dilute solutions: Soret coefficient is independent of concentration, increases with chain length (ST ~ M 0. 53) Concentrated solutions: ST is independent of chain length, decreases with concentration (ST ~ (c/c*)-0. 73) J. Rauch and W. Köhler, Phys. Rev. Lett. 88, 185901 (2002) 6

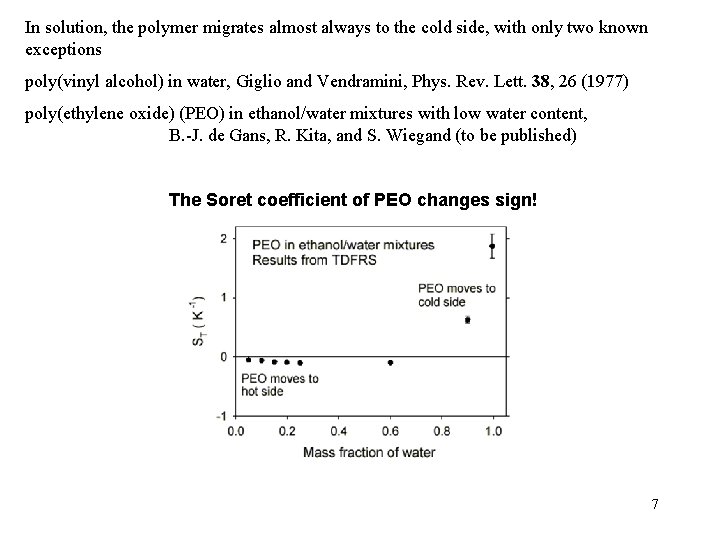
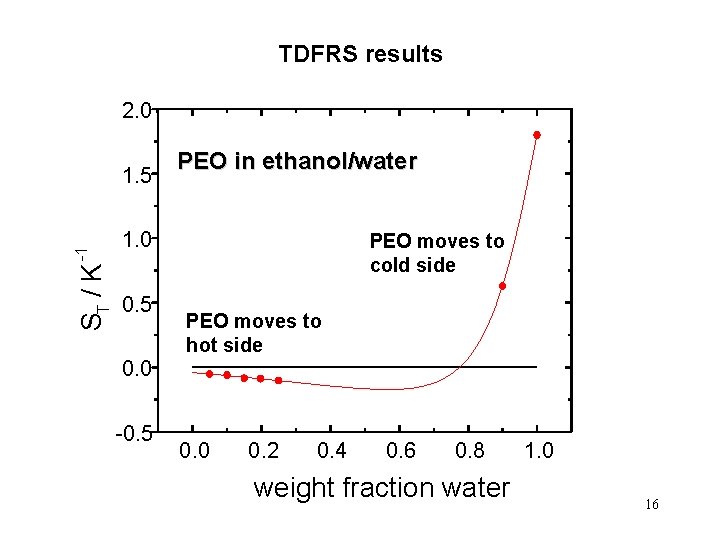
In solution, the polymer migrates almost always to the cold side, with only two known exceptions poly(vinyl alcohol) in water, Giglio and Vendramini, Phys. Rev. Lett. 38, 26 (1977) poly(ethylene oxide) (PEO) in ethanol/water mixtures with low water content, B. -J. de Gans, R. Kita, and S. Wiegand (to be published) The Soret coefficient of PEO changes sign! 7

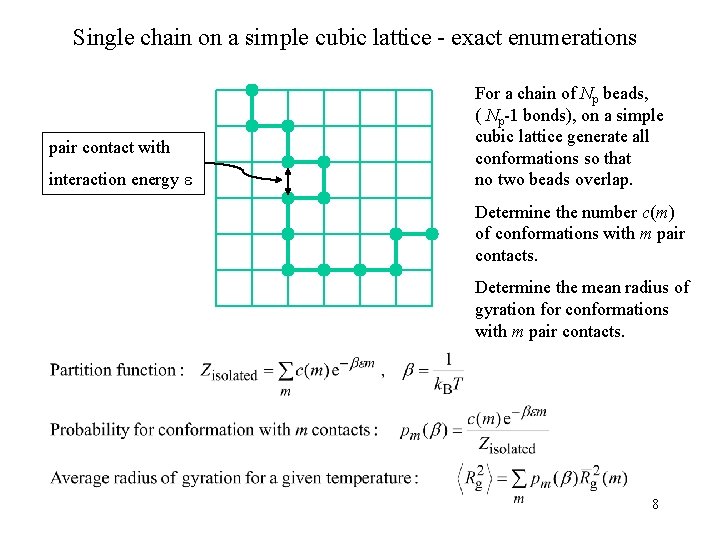
Single chain on a simple cubic lattice - exact enumerations pair contact with interaction energy For a chain of Np beads, ( Np-1 bonds), on a simple cubic lattice generate all conformations so that no two beads overlap. Determine the number c(m) of conformations with m pair contacts. Determine the mean radius of gyration for conformations with m pair contacts. 8

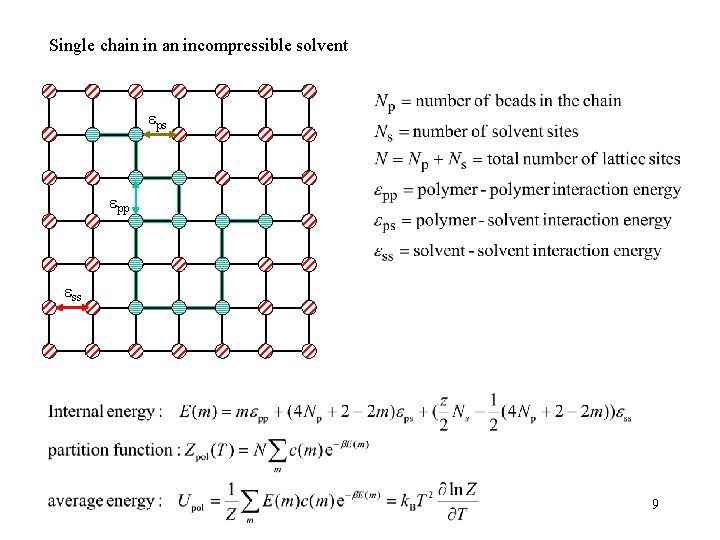
Single chain in an incompressible solvent ps pp ss 9

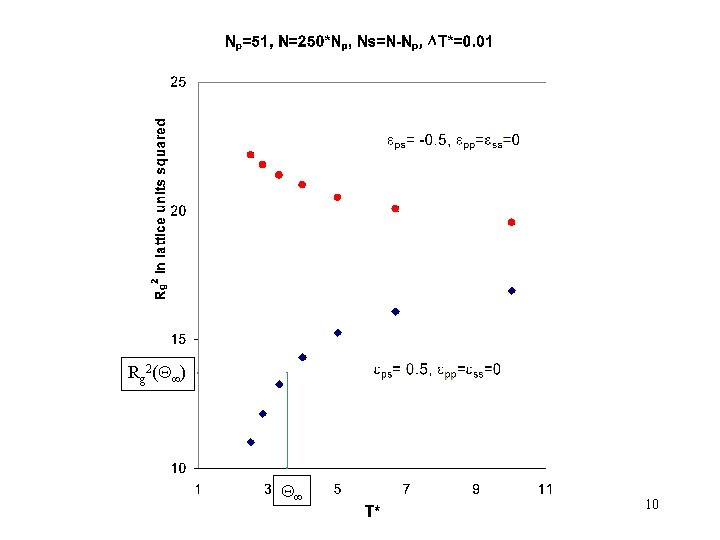
Rg 2( ) 10

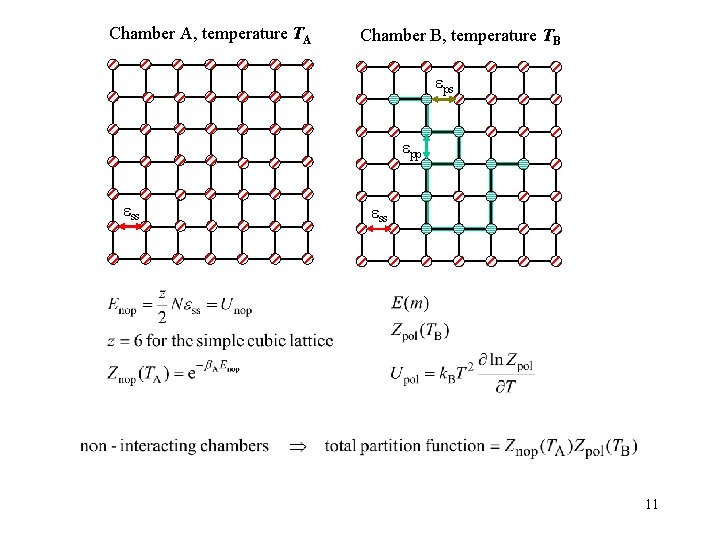
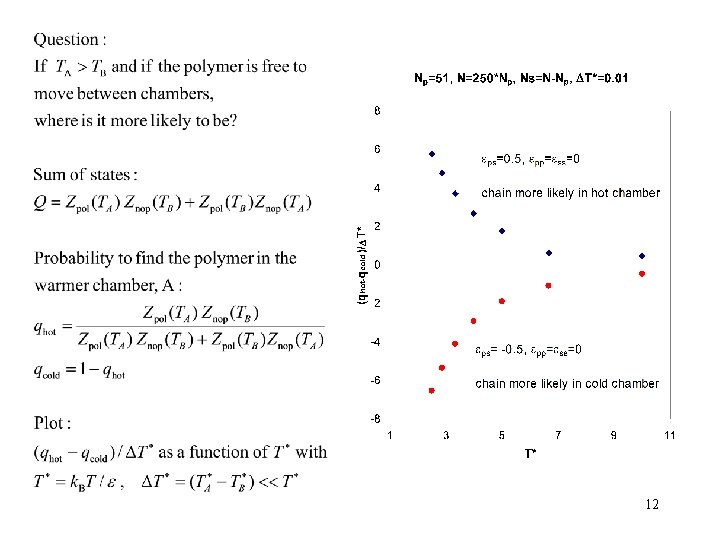
Chamber A, temperature TA Chamber B, temperature TB ps pp ss 11

12

Hence, the difference in internal energy between two boxes at the same temperature, one with and one without polymer, determines the probability to find the polymer in the warmer of two boxes at different temperatures “heat of transfer” T, Unop T, Upol TA> T B TB 13

14

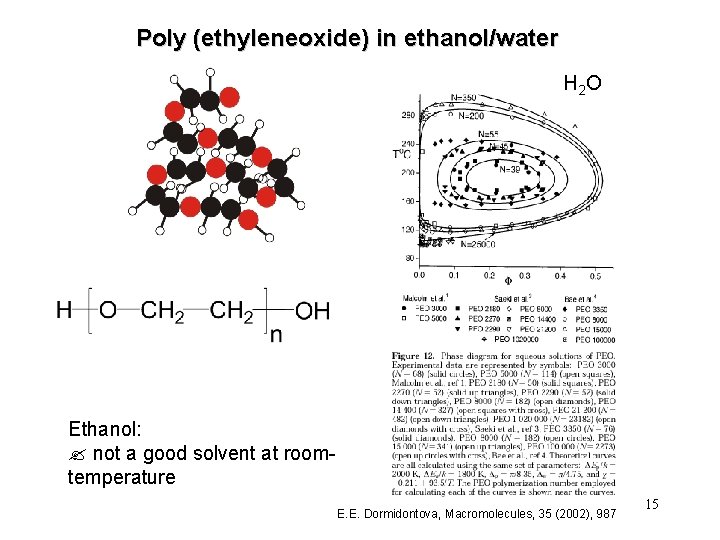
Poly (ethyleneoxide) in ethanol/water H 2 O Ethanol: ? not a good solvent at roomtemperature E. E. Dormidontova, Macromolecules, 35 (2002), 987 15

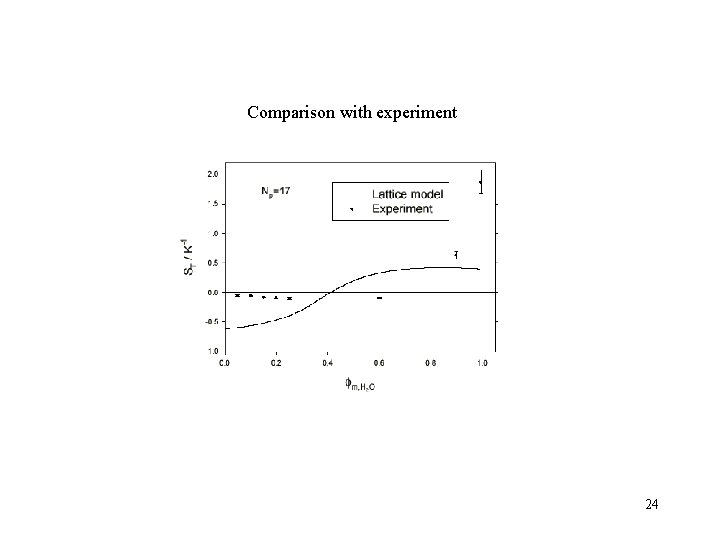
TDFRS results 2. 0 ST / K -1 1. 5 PEO in ethanol/water 1. 0 0. 5 PEO moves to cold side PEO moves to hot side 0. 0 -0. 5 0. 0 0. 2 0. 4 0. 6 0. 8 weight fraction water 1. 0 16

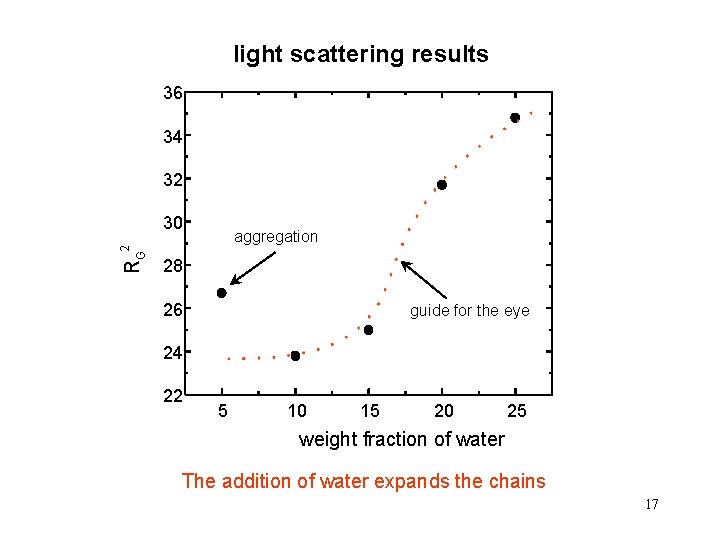
light scattering results 36 34 32 30 RG 2 aggregation 28 26 guide for the eye 24 22 5 10 15 20 25 weight fraction of water The addition of water expands the chains 17

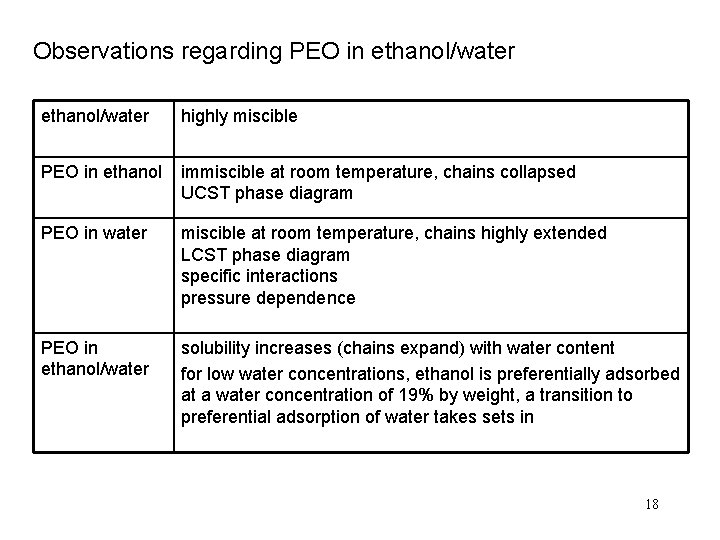
Observations regarding PEO in ethanol/water highly miscible PEO in ethanol immiscible at room temperature, chains collapsed UCST phase diagram PEO in water miscible at room temperature, chains highly extended LCST phase diagram specific interactions pressure dependence PEO in ethanol/water solubility increases (chains expand) with water content for low water concentrations, ethanol is preferentially adsorbed at a water concentration of 19% by weight, a transition to preferential adsorption of water takes sets in 18

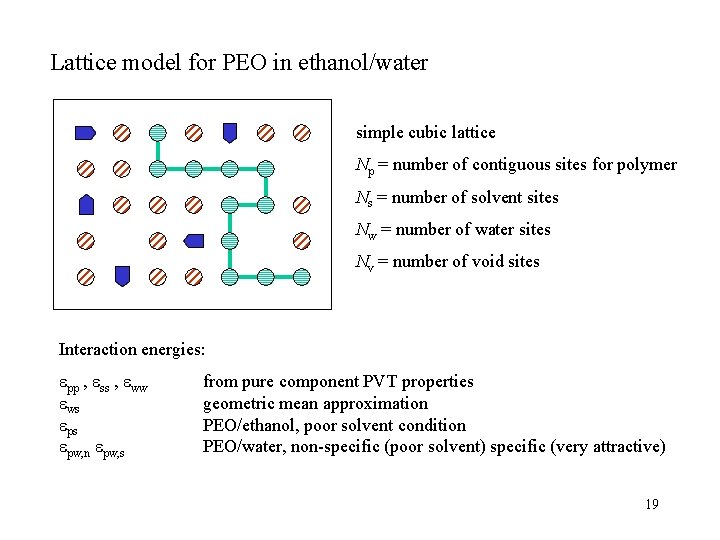
Lattice model for PEO in ethanol/water simple cubic lattice Np = number of contiguous sites for polymer Ns = number of solvent sites Nw = number of water sites Nv = number of void sites Interaction energies: pp , ss , ww ws ps pw, n pw, s from pure component PVT properties geometric mean approximation PEO/ethanol, poor solvent condition PEO/water, non-specific (poor solvent) specific (very attractive) 19

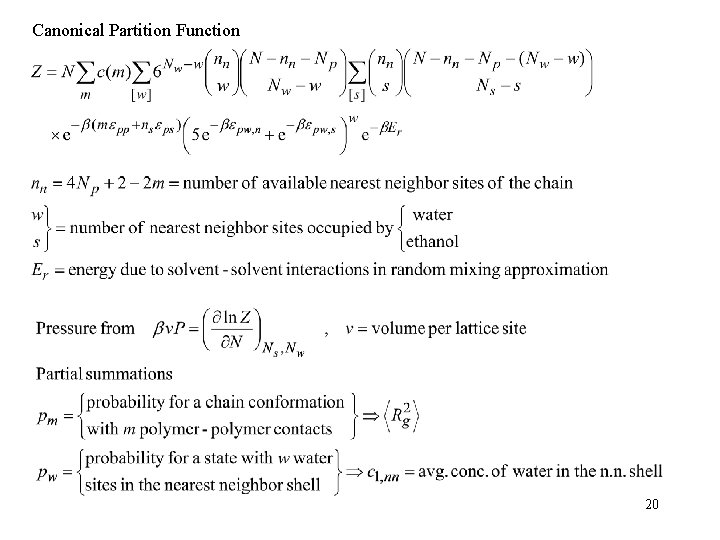
Canonical Partition Function 20

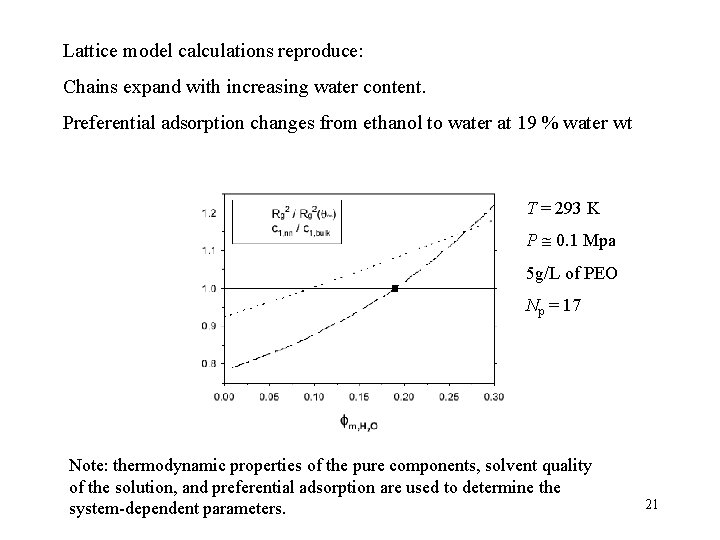
Lattice model calculations reproduce: Chains expand with increasing water content. Preferential adsorption changes from ethanol to water at 19 % water wt T = 293 K P 0. 1 Mpa 5 g/L of PEO Np = 17 Note: thermodynamic properties of the pure components, solvent quality of the solution, and preferential adsorption are used to determine the system-dependent parameters. 21

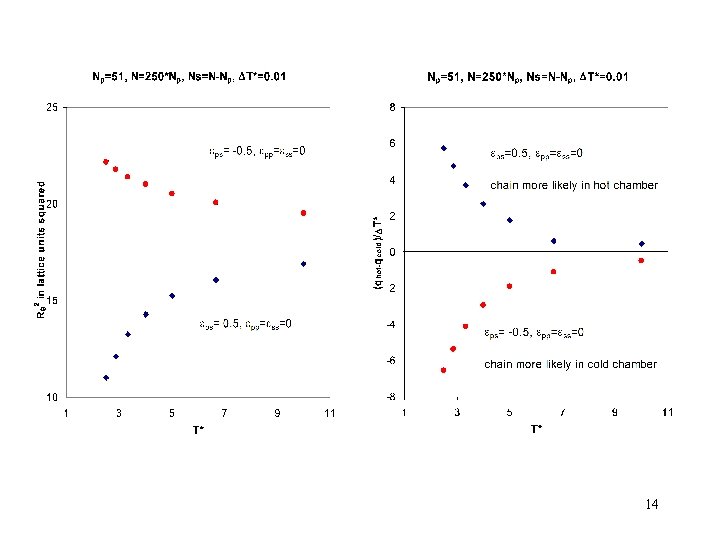
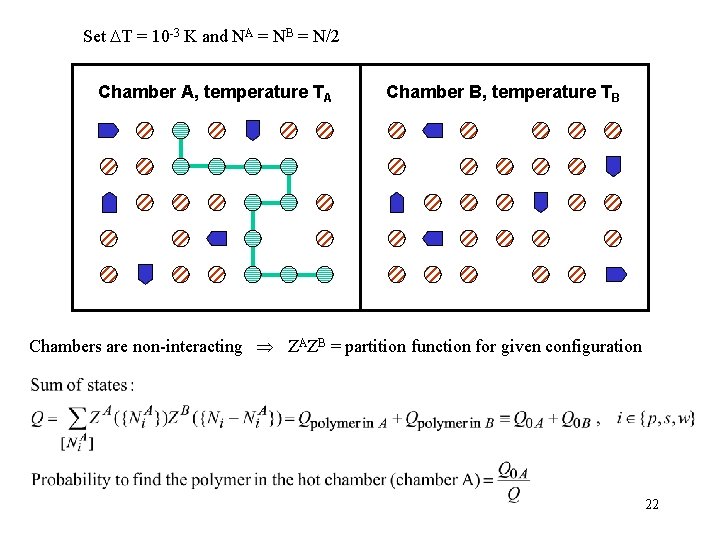
Set T = 10 -3 K and NA = NB = N/2 Chamber A, temperature TA Chamber B, temperature TB Chambers are non-interacting ZAZB = partition function for given configuration 22

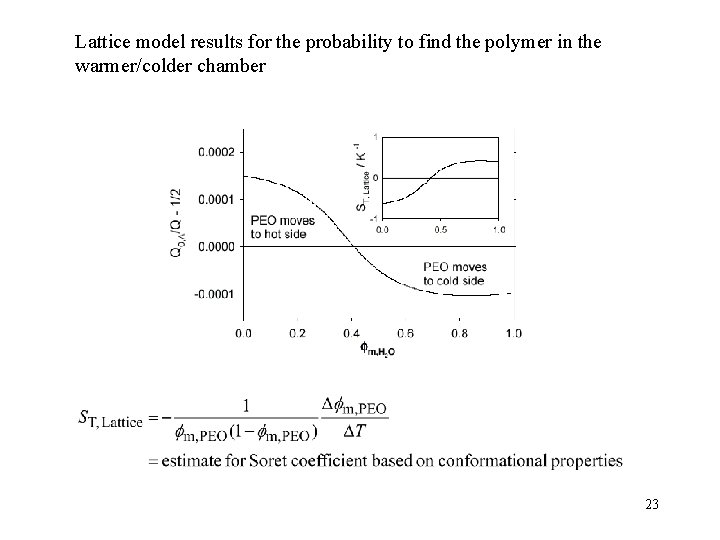
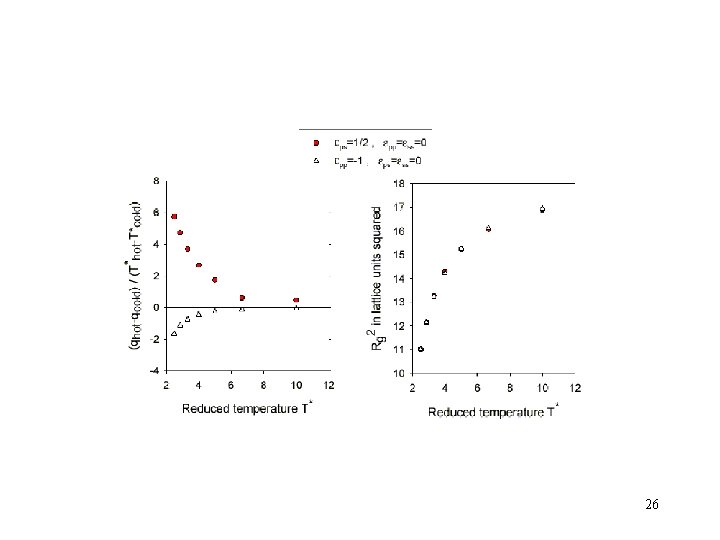
Lattice model results for the probability to find the polymer in the warmer/colder chamber 23

Comparison with experiment 24

Discussion • In general, the better the solvent quality the higher the probability to find the polymer on the cold side. • PEO moves to the cold side in ethanol/water with high water content • PEO moves to the hot side in ethanol/water with low water content • PVA moves to the hot side in water (Giglio and Vendramini, 1977) • also seen in calculations of the Soret coefficient of PEO in pure water and ethanol • In model calculations, the trend is reversed if the polymer-polymer interactions are very attractive • Preferential adsorption is an important indicator for the behavior of the Soret coefficient Acknowledgements: The authors would like to thank Mark Taylor and Simone Wiegand for many helpful discussions. Financial support through the National Science Foundation (DMR-013704), the Ohio Board of Regents, the Research Corporation (CC 5228), and the Petroleum Research Fund (#36559 GB 7) is gratefully acknowledged. 25

26
 Jutta leth
Jutta leth Proksemiikka
Proksemiikka Jutta treiber
Jutta treiber Jutta vento
Jutta vento Jutta luettmer-strathmann
Jutta luettmer-strathmann Jutta sundermann
Jutta sundermann Kasperikoti rovaniemi
Kasperikoti rovaniemi Jutta geldermann
Jutta geldermann Prof. dr. jutta rump
Prof. dr. jutta rump Jutta haslund
Jutta haslund Proksemiikka
Proksemiikka Jutta dotterweich
Jutta dotterweich Basisdokumentation psychosomatische grundversorgung
Basisdokumentation psychosomatische grundversorgung Jutta ringling
Jutta ringling Reliant capital solutions department of education
Reliant capital solutions department of education Tagged polymer
Tagged polymer Chapter 28 monomer liquid and polymer powder
Chapter 28 monomer liquid and polymer powder Example of natural polymer
Example of natural polymer Dna polymer and monomer
Dna polymer and monomer Psady reviews
Psady reviews Characteristics of polymer
Characteristics of polymer Silk is a natural protein fiber some forms of which can be
Silk is a natural protein fiber some forms of which can be Polymer additives definition
Polymer additives definition Isotactic polymer
Isotactic polymer All plant fibers share the common polymer that is
All plant fibers share the common polymer that is Surface roughness of polymer film
Surface roughness of polymer film Introduction to polymer blends
Introduction to polymer blends