THE MOLE CONCEPT THE DOZEN ANALOGY Coin Mass





- Slides: 20

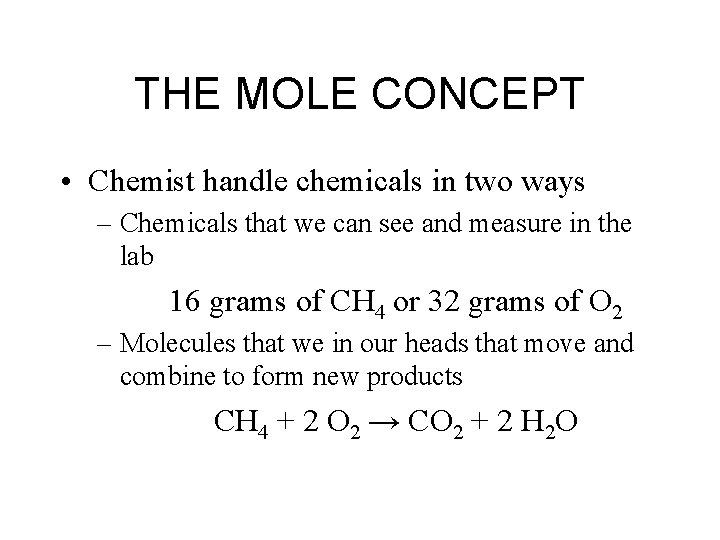
THE MOLE CONCEPT

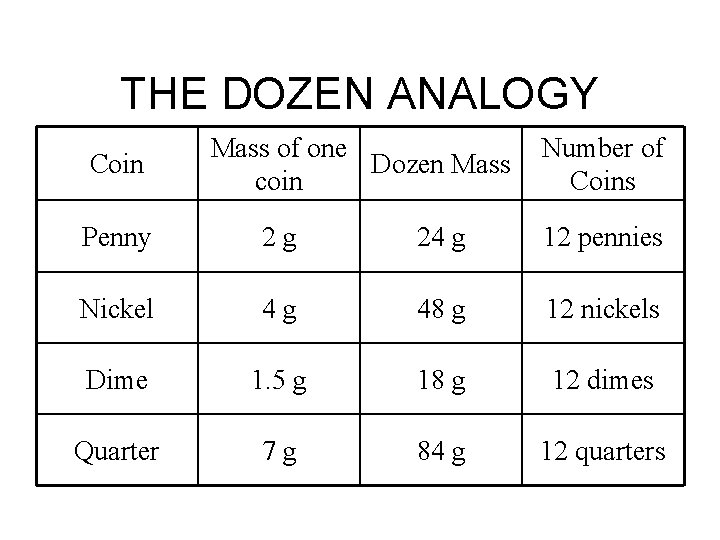
THE DOZEN ANALOGY Coin Mass of one Dozen Mass coin Number of Coins Penny 2 g 24 g 12 pennies Nickel 4 g 48 g 12 nickels Dime 1. 5 g 18 g 12 dimes Quarter 7 g 84 g 12 quarters

THE MOLE CONCEPT • Objects are measured by counting or by weight (mass) • It’s easier to measure large objects by counting – Elephants, cars, eggs • It’s easier to measure small objects by weight (mass) – Nails, rice, pellets

THE MOLE CONCEPT • Chemist handle chemicals in two ways – Chemicals that we can see and measure in the lab 16 grams of CH 4 or 32 grams of O 2 – Molecules that we in our heads that move and combine to form new products CH 4 + 2 O 2 → CO 2 + 2 H 2 O

THE MOLE CONCEPT • Through experiments we can find the relative mass of molecules – One molecule of CH 4 has a mass of 16 a. m. u. (atomic mass units) – One molecule of O 2 has a mass of 32 a. m. u. • Knowing the mass of just one molecule is not very helpful in the lab because one molecule is just too small.

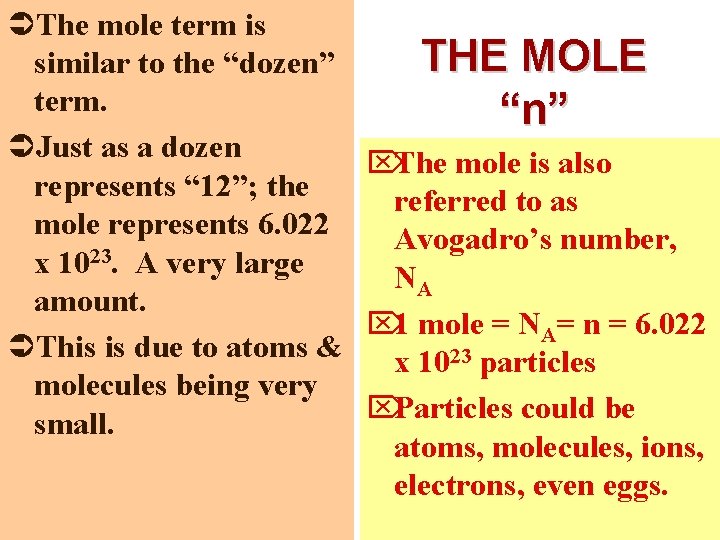
ÜThe mole term is THE MOLE similar to the “dozen” term. “n” ÜJust as a dozen ÖThe mole is also represents “ 12”; the referred to as mole represents 6. 022 Avogadro’s number, 23 x 10. A very large NA amount. Ö 1 mole = NA= n = 6. 022 ÜThis is due to atoms & x 1023 particles molecules being very Ö Particles could be small. atoms, molecules, ions, electrons, even eggs.

IMAGINE: 1 mole of any substance = 6. 02 x 1023 units of that substance. • If you spread 1 mole of un-popped popcorn kernels across the USA the entire country would be covered in popcorn to a depth of over 9 miles. • If 1 mole of marbles were spread over the surface of the Earth, our planet would be covered by a 50 -mile thick layer of marbles. • 1 mole of soft drink cans would cover the surface of the earth to a depth of over 200 miles

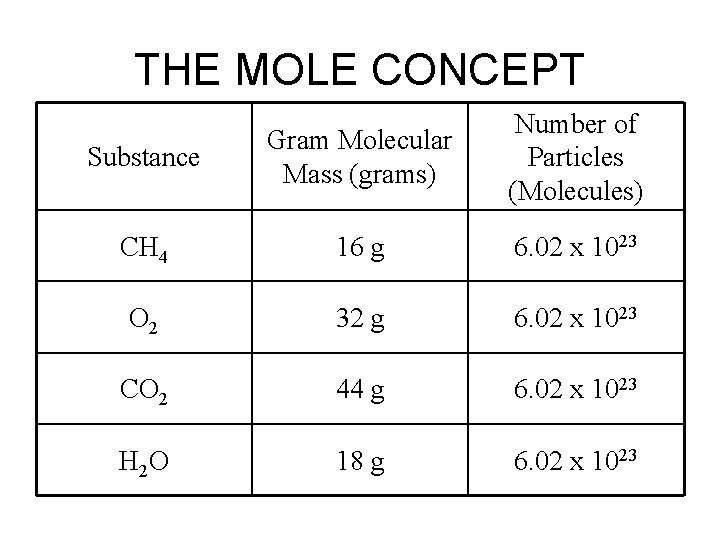
THE MOLE CONCEPT Substance Gram Molecular Mass (grams) Number of Particles (Molecules) CH 4 16 g 6. 02 x 1023 O 2 32 g 6. 02 x 1023 CO 2 44 g 6. 02 x 1023 H 2 O 18 g 6. 02 x 1023

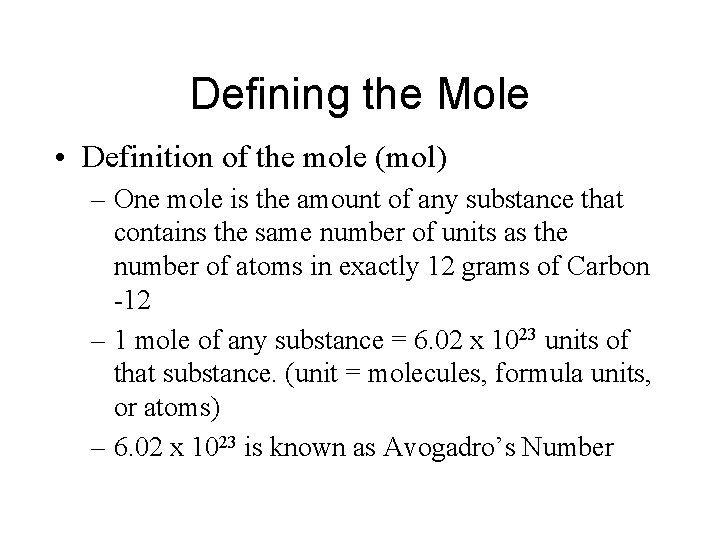
Defining the Mole • Definition of the mole (mol) – One mole is the amount of any substance that contains the same number of units as the number of atoms in exactly 12 grams of Carbon -12 – 1 mole of any substance = 6. 02 x 1023 units of that substance. (unit = molecules, formula units, or atoms) – 6. 02 x 1023 is known as Avogadro’s Number

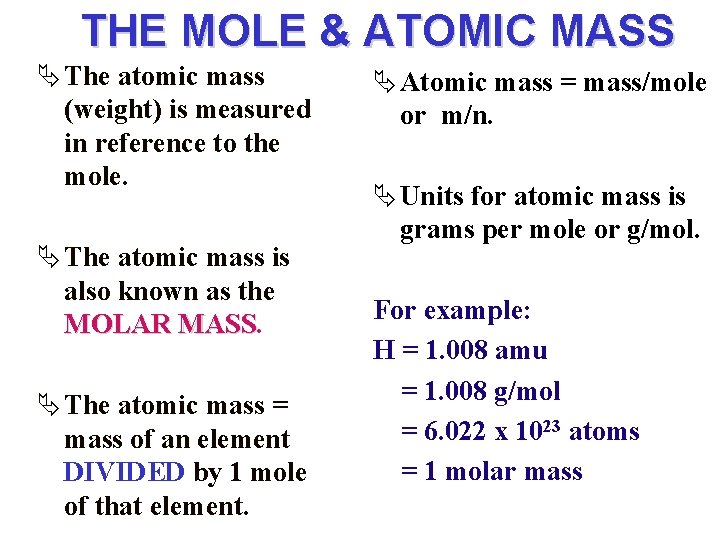
THE MOLE & ATOMIC MASS Ä The atomic mass (weight) is measured in reference to the mole. Ä The atomic mass is also known as the MOLAR MASS Ä The atomic mass = mass of an element DIVIDED by 1 mole of that element. Ä Atomic mass = mass/mole or m/n. Ä Units for atomic mass is grams per mole or g/mol. For example: H = 1. 008 amu = 1. 008 g/mol = 6. 022 x 1023 atoms = 1 molar mass

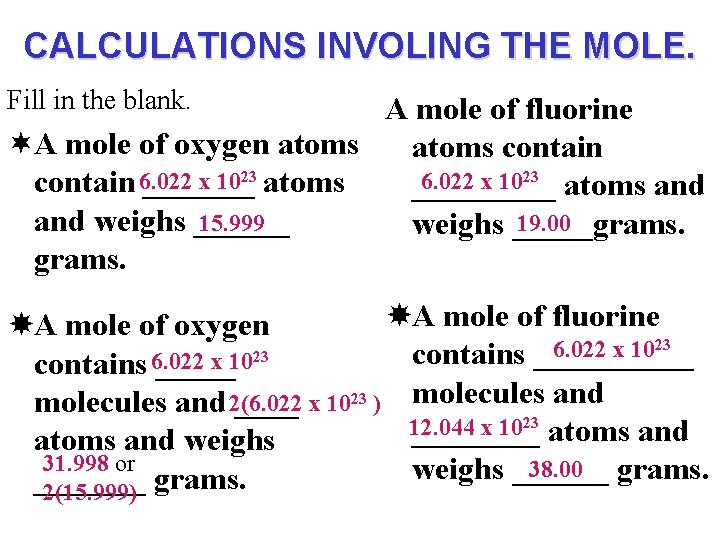
CALCULATIONS INVOLING THE MOLE. Fill in the blank. A mole of fluorine ¬A mole of oxygen atoms contain x 1023 atoms 6. 022 x 1023 atoms and contain 6. 022 _________ and weighs ______ 15. 999 19. 00 weighs _____grams. A mole of fluorine A mole of oxygen 23 6. 022 x 10 23 contains _____ x 10 contains 6. 022 _____ and molecules and 2(6. 022 ____ x 1023 ) molecules 23 12. 044 x 10 ____ atoms and weighs 31. 998 or 38. 00 grams. weighs _______ grams. 2(15. 999)

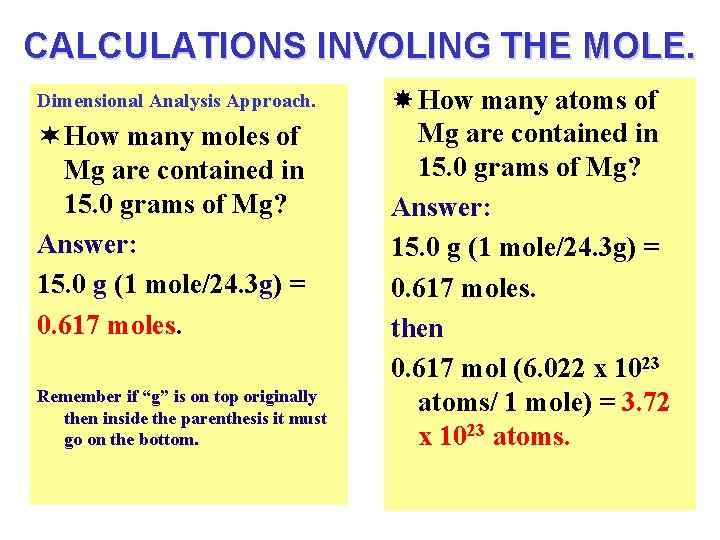
CALCULATIONS INVOLING THE MOLE. Dimensional Analysis Approach. ¬ How many moles of Mg are contained in 15. 0 grams of Mg? Answer: 15. 0 g (1 mole/24. 3 g) = 0. 617 moles. Remember if “g” is on top originally then inside the parenthesis it must go on the bottom. How many atoms of Mg are contained in 15. 0 grams of Mg? Answer: 15. 0 g (1 mole/24. 3 g) = 0. 617 moles. then 0. 617 mol (6. 022 x 1023 atoms/ 1 mole) = 3. 72 x 1023 atoms.

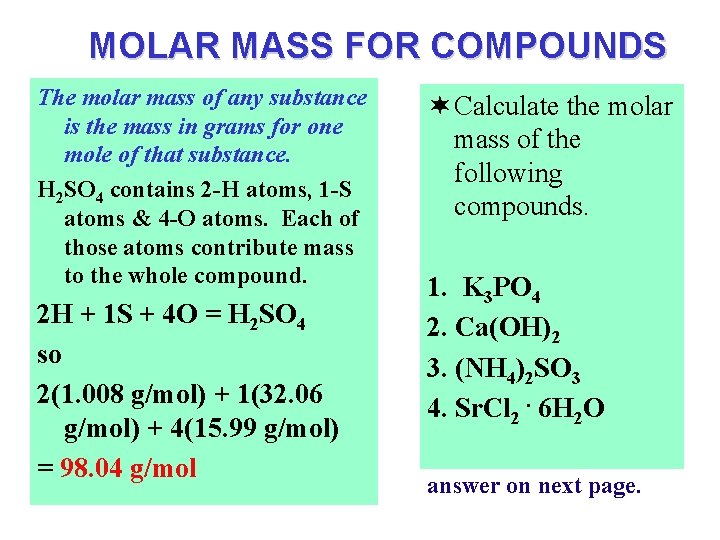
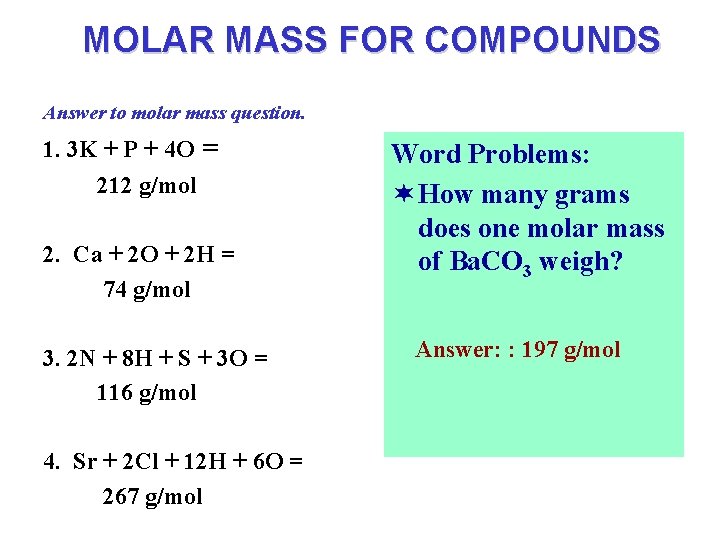
MOLAR MASS FOR COMPOUNDS The molar mass of any substance is the mass in grams for one mole of that substance. H 2 SO 4 contains 2 -H atoms, 1 -S atoms & 4 -O atoms. Each of those atoms contribute mass to the whole compound. 2 H + 1 S + 4 O = H 2 SO 4 so 2(1. 008 g/mol) + 1(32. 06 g/mol) + 4(15. 99 g/mol) = 98. 04 g/mol ¬ Calculate the molar mass of the following compounds. 1. K 3 PO 4 2. Ca(OH)2 3. (NH 4)2 SO 3 4. Sr. Cl 2. 6 H 2 O answer on next page.

MOLAR MASS FOR COMPOUNDS Answer to molar mass question. 1. 3 K + P + 4 O = 212 g/mol 2. Ca + 2 O + 2 H = 74 g/mol 3. 2 N + 8 H + S + 3 O = 116 g/mol 4. Sr + 2 Cl + 12 H + 6 O = 267 g/mol Word Problems: ¬ How many grams does one molar mass of Ba. CO 3 weigh? Answer: : 197 g/mol

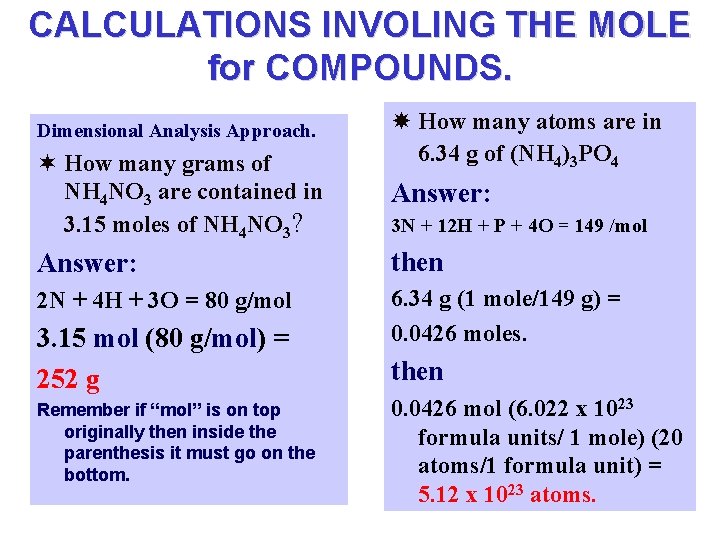
CALCULATIONS INVOLING THE MOLE for COMPOUNDS. Dimensional Analysis Approach. ¬ How many grams of NH 4 NO 3 are contained in 3. 15 moles of NH 4 NO 3? How many atoms are in 6. 34 g of (NH 4)3 PO 4 Answer: 3 N + 12 H + P + 4 O = 149 /mol Answer: then 2 N + 4 H + 3 O = 80 g/mol 6. 34 g (1 mole/149 g) = 0. 0426 moles. 3. 15 mol (80 g/mol) = 252 g Remember if “mol” is on top originally then inside the parenthesis it must go on the bottom. then 0. 0426 mol (6. 022 x 1023 formula units/ 1 mole) (20 atoms/1 formula unit) = 5. 12 x 1023 atoms.

Challenge MOLE problems. What mass of sodium will contain the same number of atoms as 100. 0 g of potassium? 100. 0 g K (1 mole K / 39. 1 g K) (6. 022 x 1023 K-atoms/1 mole K) = 1. 540 x 10 24 K-atoms. Now since atoms of K = atoms of Na 1. 540 x 1024 atoms Na (1 mol/ 6. 02 x 1023 atoms) (22. 98 g/ 1 mol) = 58. 77 g of Na

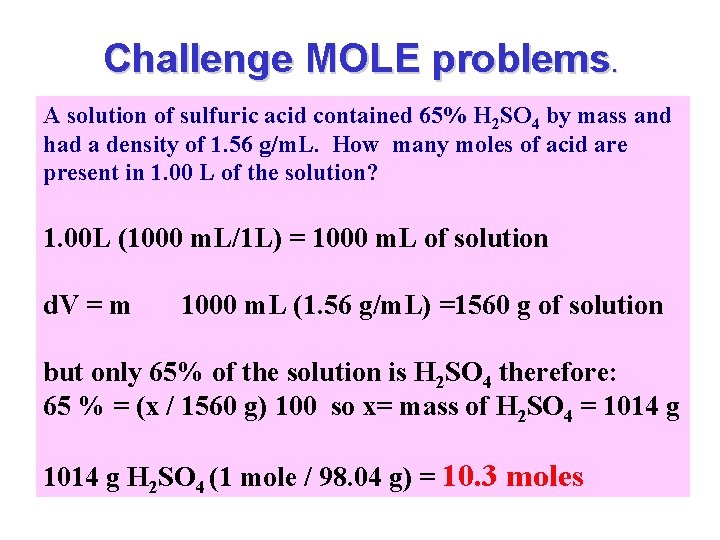
Challenge MOLE problems. A solution of sulfuric acid contained 65% H 2 SO 4 by mass and had a density of 1. 56 g/m. L. How many moles of acid are present in 1. 00 L of the solution? 1. 00 L (1000 m. L/1 L) = 1000 m. L of solution d. V = m 1000 m. L (1. 56 g/m. L) =1560 g of solution but only 65% of the solution is H 2 SO 4 therefore: 65 % = (x / 1560 g) 100 so x= mass of H 2 SO 4 = 1014 g H 2 SO 4 (1 mole / 98. 04 g) = 10. 3 moles

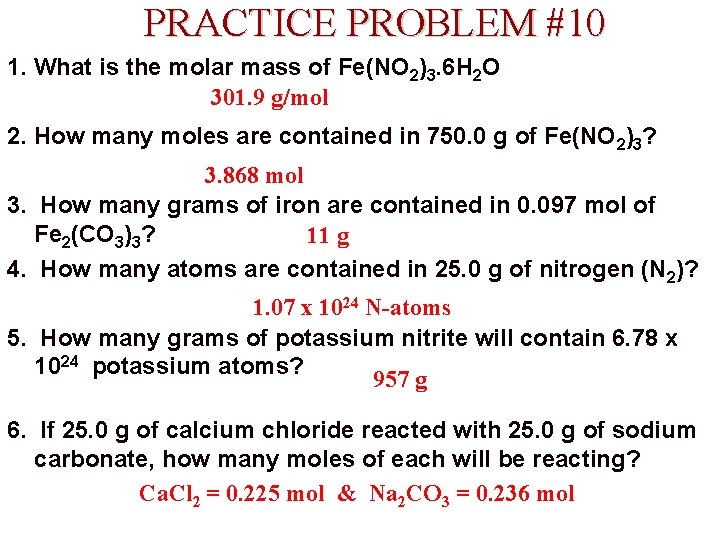
PRACTICE PROBLEM #10 1. What is the molar mass of Fe(NO 2)3. 6 H 2 O 301. 9 g/mol 2. How many moles are contained in 750. 0 g of Fe(NO 2)3? 3. 868 mol 3. How many grams of iron are contained in 0. 097 mol of Fe 2(CO 3)3? 11 g 4. How many atoms are contained in 25. 0 g of nitrogen (N 2)? 1. 07 x 1024 N-atoms 5. How many grams of potassium nitrite will contain 6. 78 x 1024 potassium atoms? 957 g 6. If 25. 0 g of calcium chloride reacted with 25. 0 g of sodium carbonate, how many moles of each will be reacting? Ca. Cl 2 = 0. 225 mol & Na 2 CO 3 = 0. 236 mol

GROUP STUDY PROBLEM #10. ______1. What is the molar mass of Ba(NO 3)2 4 H 2 O a) 185. 0 b) 261. 0 c) 333. 3 d) 1044 ______2. How many moles are contained in 54. 78 g of Cu(NO 3)2? a) 0. 2921 mol ______3. b) 3. 424 mol c) 187. 5 mol d) 0. 399 Which contains the larger number of molecules? a) 125. 0 g HCl b) 15. 0 g C 6 H 12 O 6 c) 170. 0 g of I 2 ______4. How many grams of silver are contained in 0. 754 mol of Ag. NO 3? a) 0. 488 g ______5. b) 81. 3 g c) 128 g d) 170 g 7. 0 g of nitrogen (N 2) contains a) 7. 0 atoms of N b) 0. 25 mole of N c) 3. 0 x 1023 atoms of N d) 7. 0 atomic masses of N ______6. How many atoms are in 56. 9 g of potassium nitrite? ______7. If 16. 054 g of calcium chloride reacted with 25. 004 g of sodium carbonate, how many moles of each will be reacting?

WE DIG CHEMISTRY
 Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Mole mass and mole volume relationships
Mole mass and mole volume relationships 11-2 probability and punnett squares
11-2 probability and punnett squares Mass of a dozen eggs
Mass of a dozen eggs How many in 5 dozen
How many in 5 dozen Mass of a dozen eggs
Mass of a dozen eggs Gram to gram conversion
Gram to gram conversion Mole mole factor
Mole mole factor Phosphorus with oxygen equation
Phosphorus with oxygen equation Stoichiometry mole-mole
Stoichiometry mole-mole Mole bridge chemistry
Mole bridge chemistry Mole analogy examples
Mole analogy examples Mole analogy
Mole analogy 10.1 the mole a measurement of matter
10.1 the mole a measurement of matter Mountains into molehills answer key
Mountains into molehills answer key What is mole
What is mole Mole to mass
Mole to mass How to get moles from volume
How to get moles from volume Mole to mass
Mole to mass Formula mass vs gram formula mass
Formula mass vs gram formula mass Moleformula
Moleformula