The Mole and Stoichiometry Part 2 Molar Mass







- Slides: 12

The Mole and Stoichiometry Part 2

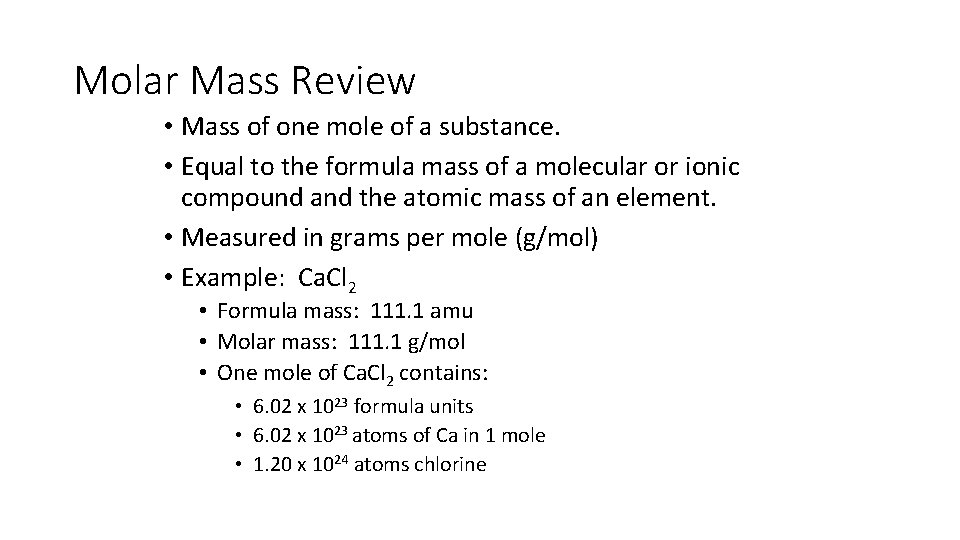
Molar Mass Review • Mass of one mole of a substance. • Equal to the formula mass of a molecular or ionic compound and the atomic mass of an element. • Measured in grams per mole (g/mol) • Example: Ca. Cl 2 • Formula mass: 111. 1 amu • Molar mass: 111. 1 g/mol • One mole of Ca. Cl 2 contains: • 6. 02 x 1023 formula units • 6. 02 x 1023 atoms of Ca in 1 mole • 1. 20 x 1024 atoms chlorine

Moles Mass • To convert between moles and mass, use the equality 1 mole= molar mass of a substance • This is your conversion factor between moles and mass

Moles Mass Examples • Example 1: What is the mass of 6. 0 moles of potassium chloride? • First calculate the molar mass of KCl • Molar mass KCl= 74. 55 g/mol 6. 0 moles of KCl 74. 55 g of KCl = 447. 3 g of KCl 1 mole of Na. Cl • Example 2: How many moles are 14. 0 grams of water? • First calculate the molar mass of water • Molar mass of H 2 O = 18. 02 g/mol 14. 0 g of H 2 O 1 mole of H 2 O 18. 02 g H 2 O = 0. 78 g H 2 O

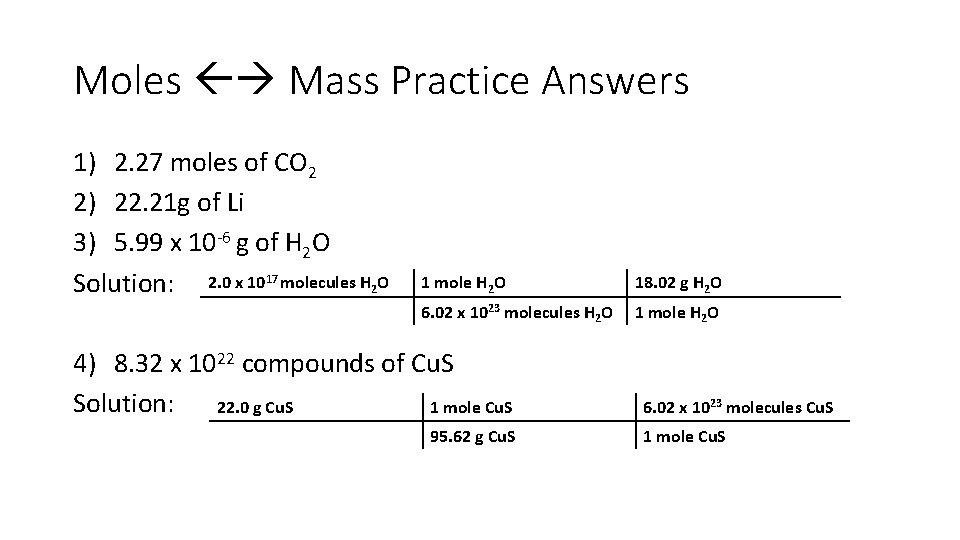
Moles Mass Practice 1. How many moles are in 100. 0 grams of carbon dioxide? 2. What is the mass of 3. 2 moles of lithium? 3. What is the mass of 2. 0 x 1017 molecules of water? (hint convert to moles first then convert to mass) 4. How many compounds are in 22. 0 g of copper (II) sulfide?

Moles Mass Practice Answers 1) 2. 27 moles of CO 2 2) 22. 21 g of Li 3) 5. 99 x 10 -6 g of H 2 O Solution: 2. 0 x 1017 molecules H 2 O 1 mole H 2 O 18. 02 g H 2 O 6. 02 x 1023 molecules H 2 O 1 mole H 2 O 4) 8. 32 x 1022 compounds of Cu. S Solution: 22. 0 g Cu. S 1 mole Cu. S 95. 62 g Cu. S 6. 02 x 1023 molecules Cu. S 1 mole Cu. S

Moles Volume • To convert between moles and volume, use the equality 1 mole= 22. 4 L of gas @ STP • This conversion is for Gases ONLY • STP stands for Standard Temperature and Pressure • Standard temperature is 273 K • Standard pressure is 1 atm

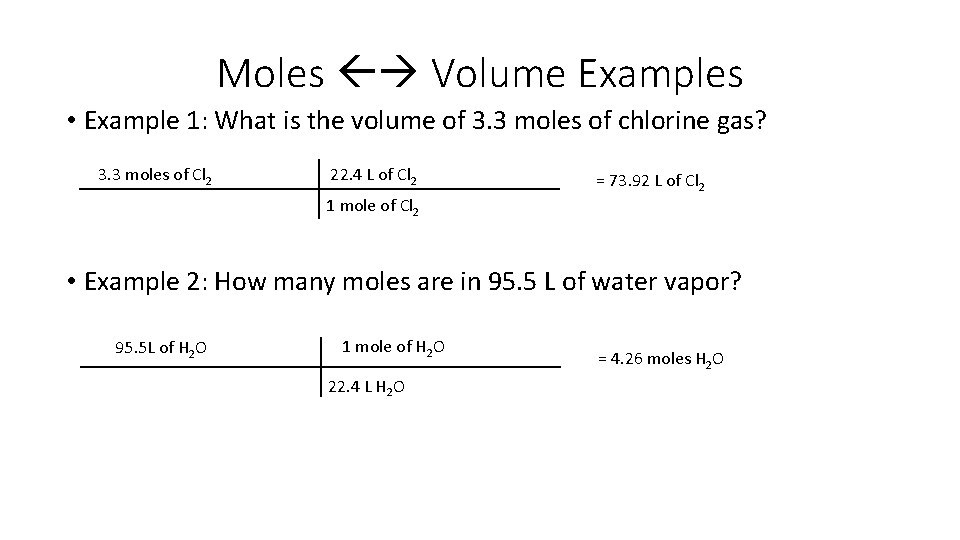
Moles Volume Examples • Example 1: What is the volume of 3. 3 moles of chlorine gas? 3. 3 moles of Cl 2 22. 4 L of Cl 2 = 73. 92 L of Cl 2 1 mole of Cl 2 • Example 2: How many moles are in 95. 5 L of water vapor? 95. 5 L of H 2 O 1 mole of H 2 O 22. 4 L H 2 O = 4. 26 moles H 2 O

Moles Volume Practice 1. How many moles are in 56. 7 L of carbon dioxide? 2. What is the volume of 0. 50 moles of oxygen gas? 3. What is the volume of 2. 0 x 1026 molecules of water vapor? (hint convert to moles first then convert to liters) 4. How many diatomic molecules are in 22. 4 L of sulfur dioxide?

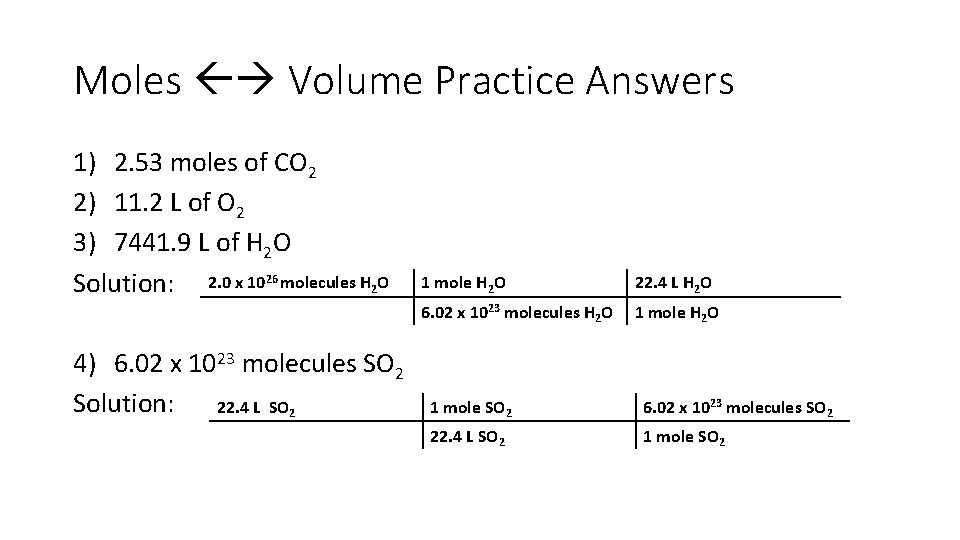
Moles Volume Practice Answers 1) 2. 53 moles of CO 2 2) 11. 2 L of O 2 3) 7441. 9 L of H 2 O Solution: 2. 0 x 1026 molecules H 2 O 4) 6. 02 x 1023 molecules SO 2 Solution: 22. 4 L SO 2 1 mole H 2 O 22. 4 L H 2 O 6. 02 x 1023 molecules H 2 O 1 mole SO 2 6. 02 x 1023 molecules SO 2 22. 4 L SO 2 1 mole SO 2

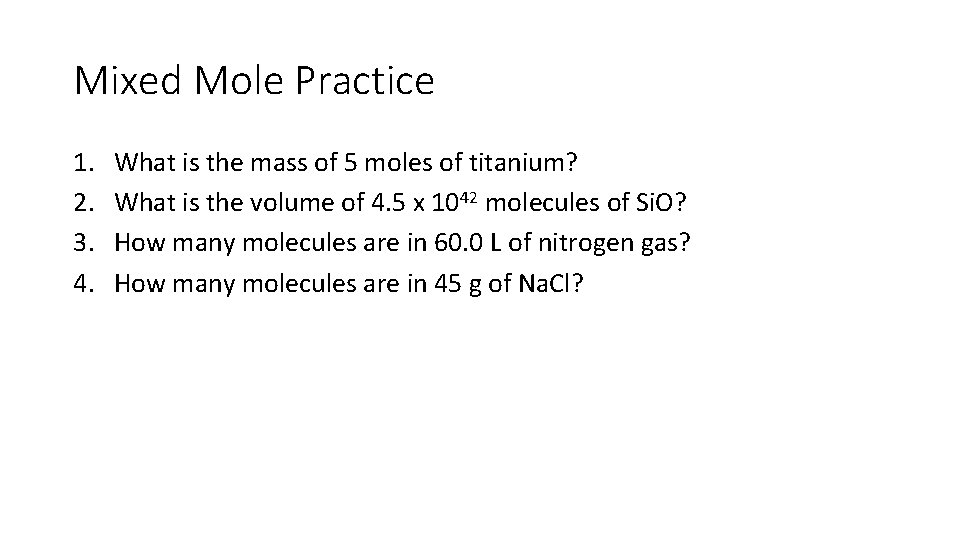
Mixed Mole Practice 1. 2. 3. 4. What is the mass of 5 moles of titanium? What is the volume of 4. 5 x 1042 molecules of Si. O? How many molecules are in 60. 0 L of nitrogen gas? How many molecules are in 45 g of Na. Cl?

Mixed Mole Practice 1) 2) 3) 4) 239. 4 g Ti 1. 67 x 1020 L of Si. O 1. 61 x 1020 molecules of N 2 4. 64 x 1023 molecule Na. Cl
 Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Mole problem
Mole problem Atomic mass
Atomic mass Stoichiometry mole-mole
Stoichiometry mole-mole Mole-mass-volume relationships
Mole-mass-volume relationships Stoichiometry molar mass
Stoichiometry molar mass How to solve stoichiometric calculations
How to solve stoichiometric calculations Gram to moles
Gram to moles Unit of molar mass
Unit of molar mass Molar mass unit
Molar mass unit The mole bridge
The mole bridge How to find mol from mass
How to find mol from mass Mass number formula
Mass number formula