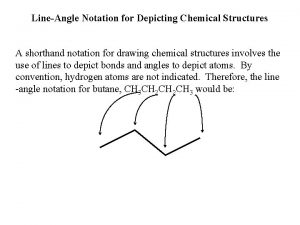
The Chemical Equation is a shorthand expression for

















- Slides: 24

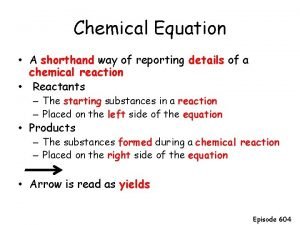
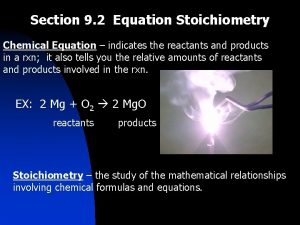
The Chemical Equation is a shorthand expression for a chemical change or reaction CHAPTER 8 CHEMICAL EQUATIONS

The Arrow • The divides the reactants from the products • Ex. Reactant(s) Product(s)

The Plus • The + sign is placed between reactants or products showing that they are separate until the reaction occurs • Reactant + Reactant Product + Product

Heat • The sign indicates that HEAT was added to the reaction • This is usually placed over or under the arrow.

States of matter in chemical equations • The physical state of the reactants and products are given by: • (l) for liquid; • (s) solid; • (g) gas; • (aq) substances in aqueous solution. • You must be told this to know which state to write. *

Law of Conservation of Matter BALANCING REACTIONS

Coefficients • Coefficients are placed in front of a substance to balance the equation and to indicate the number of units of each substance reacting or being produced

8. 2 Writing Balanced Equations • According to the Law of Conservation of Matter you must have the same number of each kind of atom before and after the reaction occurs; You can use COEFFICIENTS to achieve this you cannot change the SUBSCRIPTS • 1. Write the word equation for what is happening • 2. Write the charge balanced compounds; molecular formulas for each substance in the rxn • 3. Write the unbalanced reaction • 4. Use COEFFICIENTS to balance the reaction • 5. Check to see that there are the same number of each kind of atom on the reactant and product side.

Types of Reactions Most reactions fit into some general form or type of reaction. There are 100 s of types; we’ll look at 5!

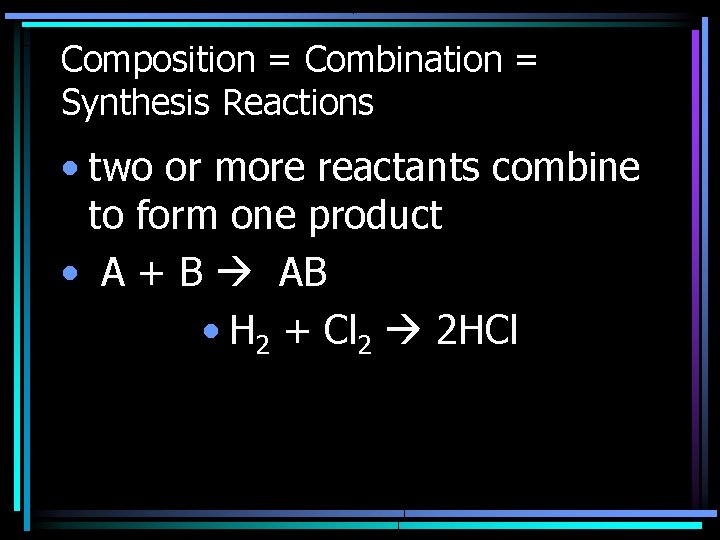
Composition = Combination = Synthesis Reactions • two or more reactants combine to form one product • A + B AB • H 2 + Cl 2 2 HCl

Zn + S Zn. S ZINC COMBINES WITH SULFUR (: 31)

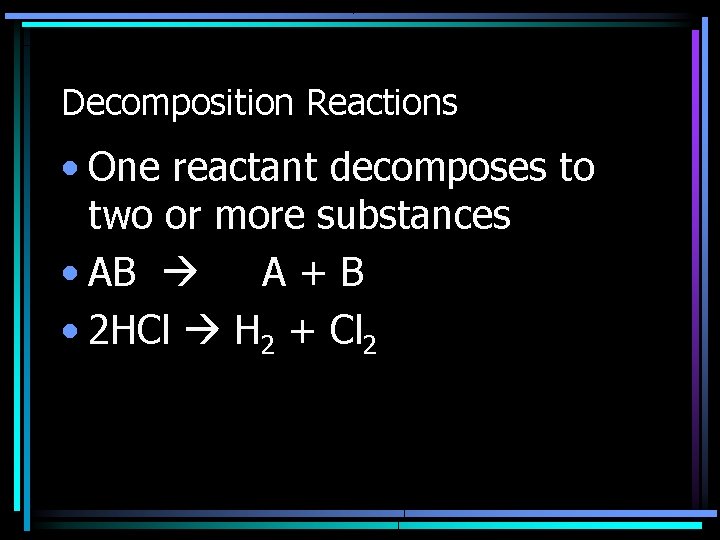
Decomposition Reactions • One reactant decomposes to two or more substances • AB A+B • 2 HCl H 2 + Cl 2

H 2 O 2 H 2 O + O 2 (: 35) ELEPHANT TOOTHPASTE

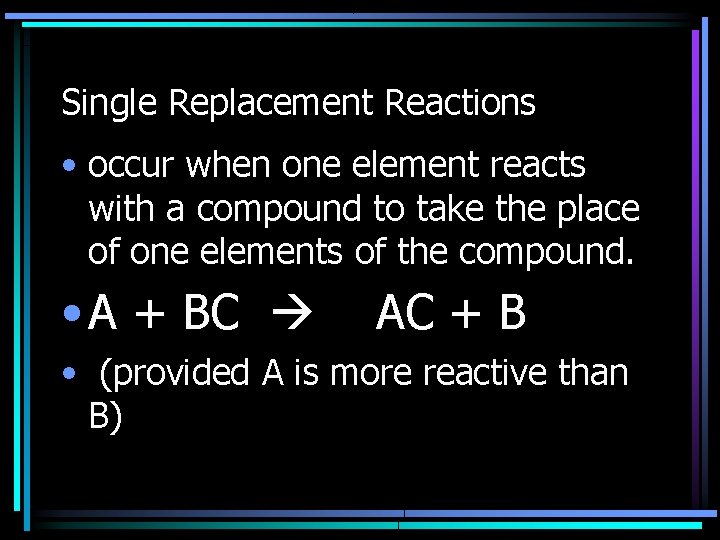
Single Replacement Reactions • occur when one element reacts with a compound to take the place of one elements of the compound. • A + BC AC + B • (provided A is more reactive than B)

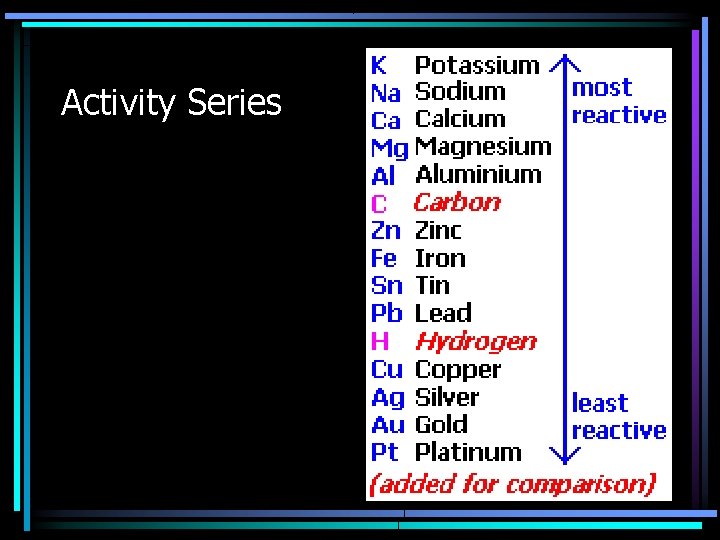
Activity Series

Zn + 2 HCl Zn. Cl 2 + H 2 LINK TO SINGLE REPLACEMENT REACTION (1: 55)

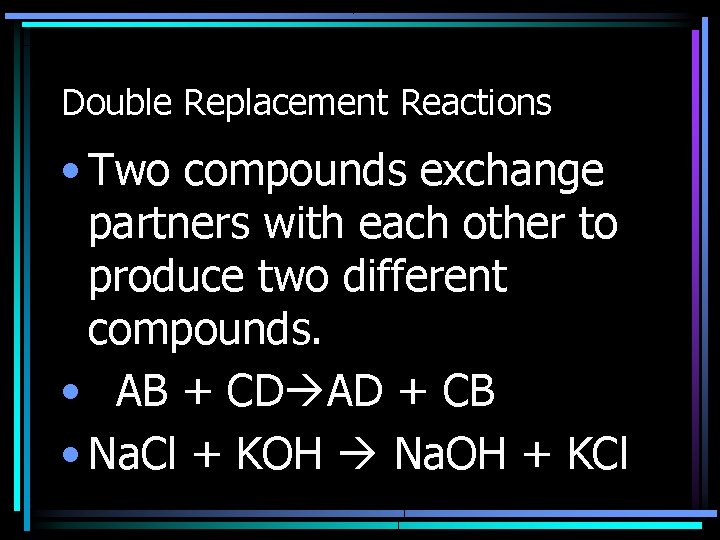
Double Replacement Reactions • Two compounds exchange partners with each other to produce two different compounds. • AB + CD AD + CB • Na. Cl + KOH Na. OH + KCl

Video Review (2: 42) DOUBLE REPLACEMENT

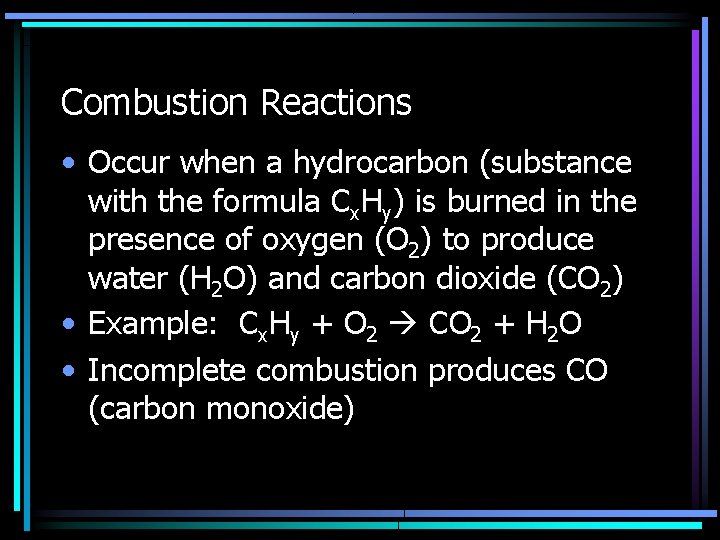
Combustion Reactions • Occur when a hydrocarbon (substance with the formula Cx. Hy) is burned in the presence of oxygen (O 2) to produce water (H 2 O) and carbon dioxide (CO 2) • Example: Cx. Hy + O 2 CO 2 + H 2 O • Incomplete combustion produces CO (carbon monoxide)

Combustion and the Hindenburg DON’T TRY THIS AT HOME (1: 31)

A video review (1: 36) CHEMICAL REACTION TYPES

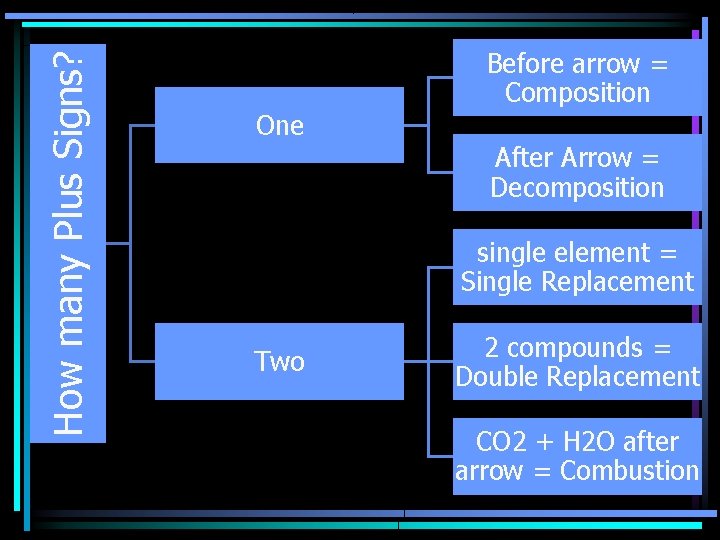
How many Plus Signs? Before arrow = Composition One After Arrow = Decomposition single element = Single Replacement Two 2 compounds = Double Replacement CO 2 + H 2 O after arrow = Combustion

8. 4 Types of Chemical Reactions • Combination, Composition, or Synthesis Reaction --two or more reactants combine to form one product A + B AB • Decomposition Reaction -- One reactant decomposes to two or more substances AB A+B • Single Replacement Reactions occur when one element reacts with a compound to take the place of one elements of the compound. Usually a metal replaces another according to the activity series on p. 161 A + BC AC + B (provided A is more reactive than B) but if not, then X + YZ no rxn (if X is not more reactive than Y) • Double Replacement Rxn. Two compounds exchange partners with each other to produce two different cds. AB + CD AD + CB (does not rely on the activity series) Not all reactions take place here either but you are not responsible to know which will and will not.

8. 5 Heat in Chemical Reactions • As discussed before: some rxns produce heat (feel hot) and others require heat (feel cold) • Exothermic reactions are ones that give off energy in the form of heat and the amount of energy is written as a product • Example of Exothermic: H 2 + Cl 2 2 HCl + 185 k. J • An Endothermic reaction requires energy and feels cold. Here, heat is written as a reactant since it is required to make the reaction occur • Example of Endothermic: N 2 + O 2 + 181 k. J 2 NO
 Shorthand expression
Shorthand expression A chemists shorthand way of representing chemical reaction
A chemists shorthand way of representing chemical reaction A chemist shorthand way of representing chemical reaction
A chemist shorthand way of representing chemical reaction Chemist shorthand way of representing chemical reaction
Chemist shorthand way of representing chemical reaction Chemical shorthand
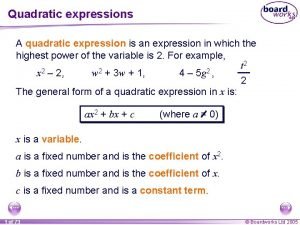
Chemical shorthand Quadratic equation examples
Quadratic equation examples 150 000 in scientific notation
150 000 in scientific notation Shorthand assignment operator
Shorthand assignment operator Scientific notation is a shorthand way of writing really
Scientific notation is a shorthand way of writing really Electron geometry
Electron geometry Medical short hand
Medical short hand Percentile shorthand
Percentile shorthand Palm pilot shorthand
Palm pilot shorthand Most letter formats require single spacing.
Most letter formats require single spacing. Noble gas shorthand
Noble gas shorthand Shorthand electron configuration for potassium
Shorthand electron configuration for potassium Electron configuraton
Electron configuraton Tin electron configuration
Tin electron configuration Dividing polynomials synthetic division
Dividing polynomials synthetic division Shorthand notation
Shorthand notation Binary shorthand for base 2
Binary shorthand for base 2 Shorthand symbols
Shorthand symbols Binary shorthand
Binary shorthand Jim crow shorthand for separation
Jim crow shorthand for separation Shorthand electron configuration for arsenic
Shorthand electron configuration for arsenic