LESSON 2 1 CHEMICAL SHORTHAND CHEMICAL FORMULAS w













- Slides: 16

LESSON 2. 1 CHEMICAL SHORTHAND

CHEMICAL FORMULAS w. A chemical formula of a compound tells how much of each element is present. w. Examples: H₂O shows two atoms of hydrogen and one atom of oxygen

OXIDATION NUMBERS w The oxidation number is the number of electrons an atom gains, loses, or shares, when bonding with another atom. (This can also be called the valence number. ) w Example: an atom of Na has a charge of ⁺ 1, so its oxidation number is also ⁺ 1.

w. If an element has more than one oxidation number, you use a Roman Numeral in the name of the compound to indicate the oxidation number. w. Example: w. Copper(I) Cu⁺ w. Copper(II) Cu⁺²

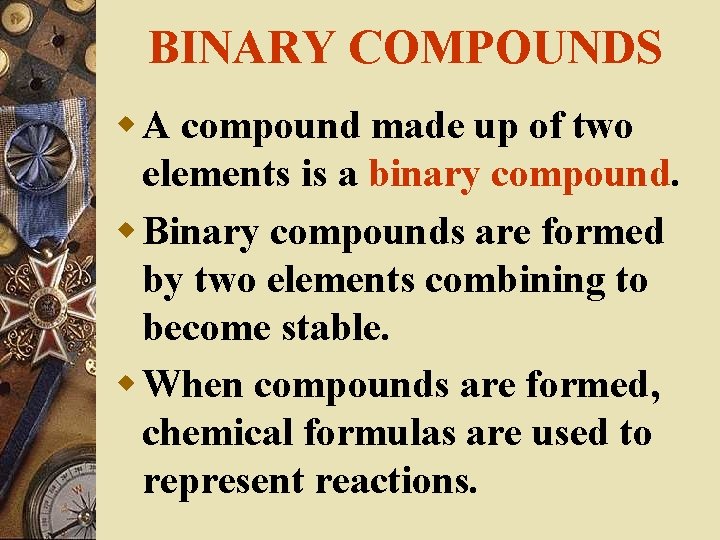
BINARY COMPOUNDS w A compound made up of two elements is a binary compound. w Binary compounds are formed by two elements combining to become stable. w When compounds are formed, chemical formulas are used to represent reactions.

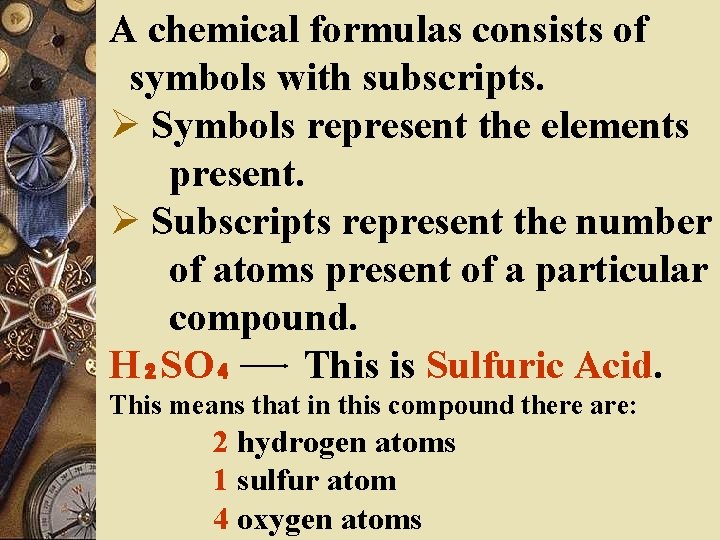
A chemical formulas consists of symbols with subscripts. Ø Symbols represent the elements present. Ø Subscripts represent the number of atoms present of a particular compound. H₂SO₄ This is Sulfuric Acid. This means that in this compound there are: 2 hydrogen atoms 1 sulfur atom 4 oxygen atoms

LET’S PRACTICE Give the name and number of atoms present in the following compounds. 1. H₂O Hydrogen oxide (Water) 2. HNO₃ Nitric acid 3. Na. Cl Sodium chloride 4. K₂SO₄ Potassium sulfate 5. Al₂O₃ Aluminum oxide

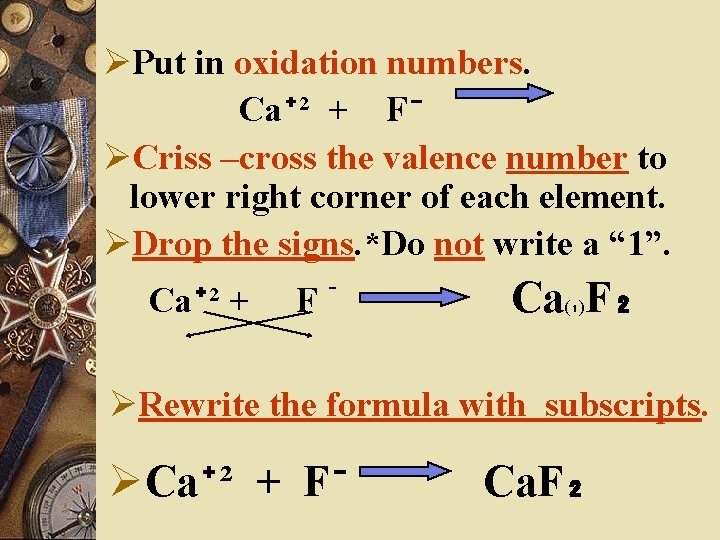
STEPS TO WRITE A BINARY FORMULA w. Write the symbol for the element with the positive oxidation number. w. Write the symbol for the element with the negative oxidation number. w. Ca + F

ØPut in oxidation numbers. Ca⁺² + F⁻ ØCriss –cross the valence number to lower right corner of each element. ØDrop the signs. *Do not write a “ 1”. Ca⁺² + F⁻ Ca(₁)F₂ ØRewrite the formula with subscripts. ØCa⁺² + F⁻ Ca. F₂

When you have oxidation numbers that are the same but have opposite signs the numbers will cancel each other out. No subscripts need to be written. EX: Na⁺ + Cl ⁻ Ba ⁺² + S ⁻² Na. Cl Ba. S

LET’S PRACTICE u Lithium + oxygen Li + O Li⁺+ O ⁻² Li⁺ + O ⁻² Li + O Li₂O(1) Li₂O

MORE PRACTICE w Magnesium + nitrogen w Lithium + sulfur w Calcium + phosphorus w Potassium + chlorine w Barium + oxide

NAMING BINARY COMPOUNDS w Write the name of the first element. w Write the root of the name of the second element. w Add the suffix –ide to the root. w Example: Na₂S sodium sulfide w Mg. O magnesium oxide

NAMING COMPOUNDS: 1. Magnesium + Nitrogen 2. Lithium + Sulfur 3. Calcium + Phosphorus 4. Potassium + Chlorine 5. Barium + Oxygen

POLYATOMIC IONS w. A polyatomic ion is a group of positively or negatively charged covalently bonded atoms. w. Naming: say the name of the element and polyatomic ion w. Example: K₂SO₄ is potassium sulfate

WRITING FORMULAS FOR POLYATOMIC IONS w. Follow the rules for binary compounds w. Add parentheses around the polyatomic ion formula if there is more than one ion needed w. Example: K₂CO₃ -one carbonate ion w. Si(CO₃)₂ -two carbonate ions