Electron Configuration Chapter 5 Electrons have 3 levels







- Slides: 28

Electron Configuration Chapter 5

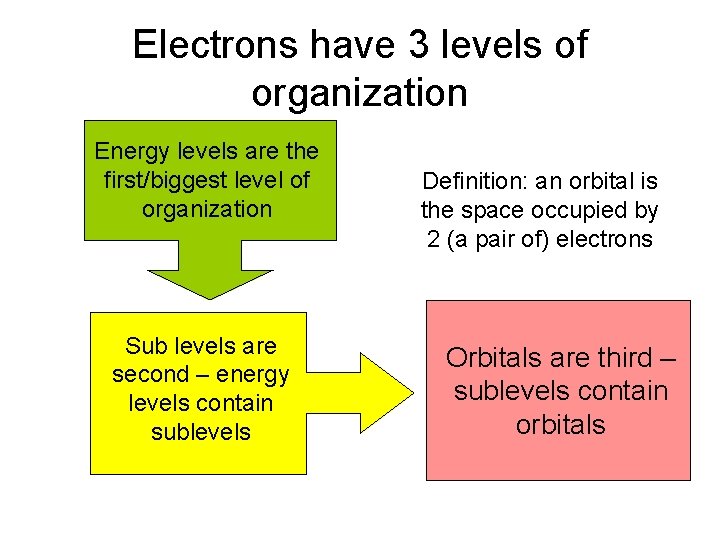
Electrons have 3 levels of organization Energy levels are the first/biggest level of organization Sub levels are second – energy levels contain sublevels Definition: an orbital is the space occupied by 2 (a pair of) electrons Orbitals are third – sublevels contain orbitals

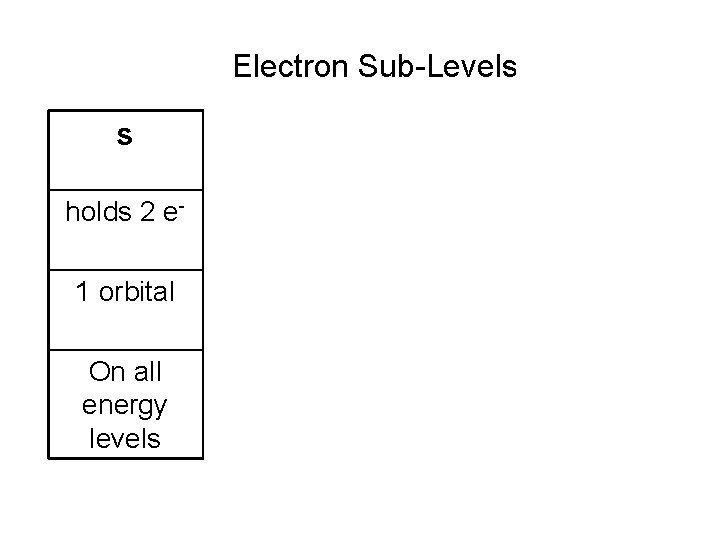
Electron Sub-Levels s holds 2 e 1 orbital On all energy levels p d f holds 6 e- holds 10 e- holds 14 e 3 orbitals 5 orbitals 7 orbitals On energy levels 2 levels 3 -6 levels 4 -5 and up

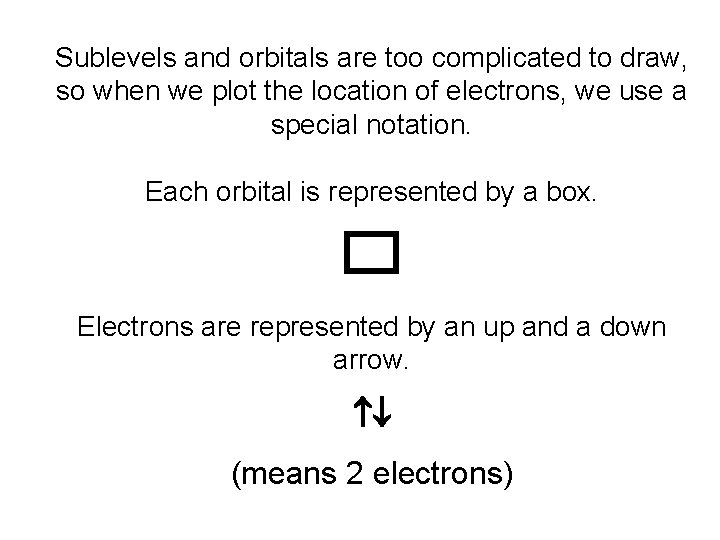
Sublevels and orbitals are too complicated to draw, so when we plot the location of electrons, we use a special notation. Each orbital is represented by a box. Electrons are represented by an up and a down arrow. (means 2 electrons)

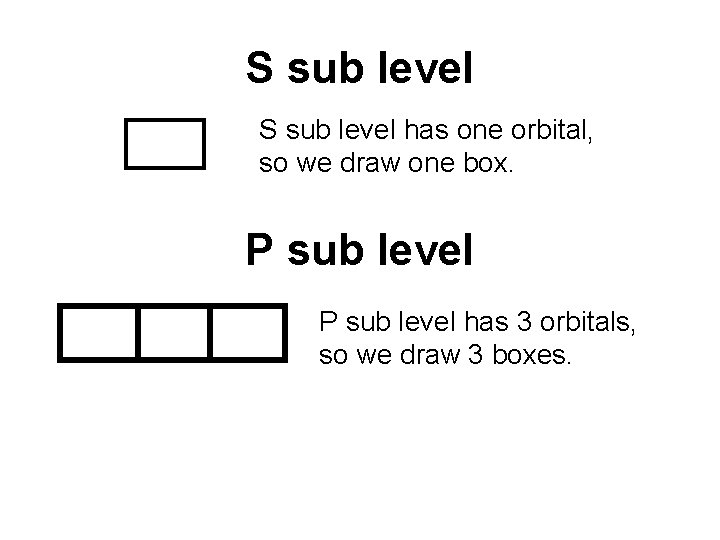
S sub level has one orbital, so we draw one box. P sub level has 3 orbitals, so we draw 3 boxes.

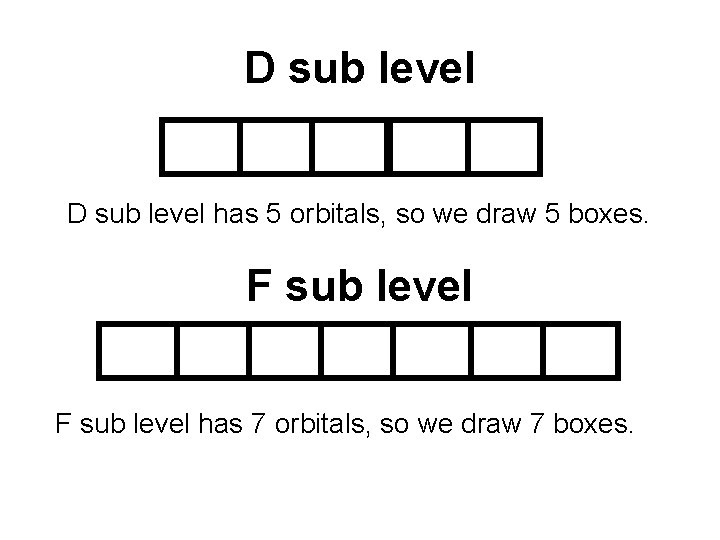
D sub level has 5 orbitals, so we draw 5 boxes. F sub level has 7 orbitals, so we draw 7 boxes.

Orbital and sublevel information is like a map, telling you where an electron can be found in an atom. There are rules that govern why an electron will be in one sub-level rather than another. Next are the directions for filling in electrons in sublevels and orbitals.

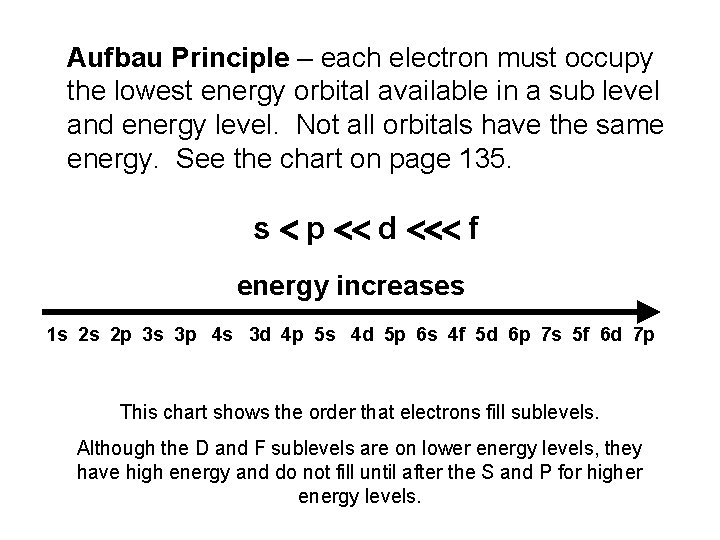
Aufbau Principle – each electron must occupy the lowest energy orbital available in a sub level and energy level. Not all orbitals have the same energy. See the chart on page 135. s p d f energy increases 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 4 f 5 d 6 p 7 s 5 f 6 d 7 p This chart shows the order that electrons fill sublevels. Although the D and F sublevels are on lower energy levels, they have high energy and do not fill until after the S and P for higher energy levels.

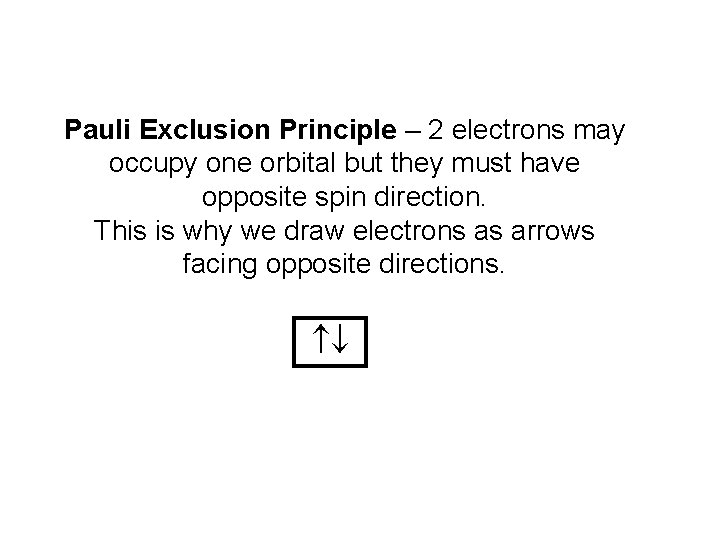
Pauli Exclusion Principle – 2 electrons may occupy one orbital but they must have opposite spin direction. This is why we draw electrons as arrows facing opposite directions.

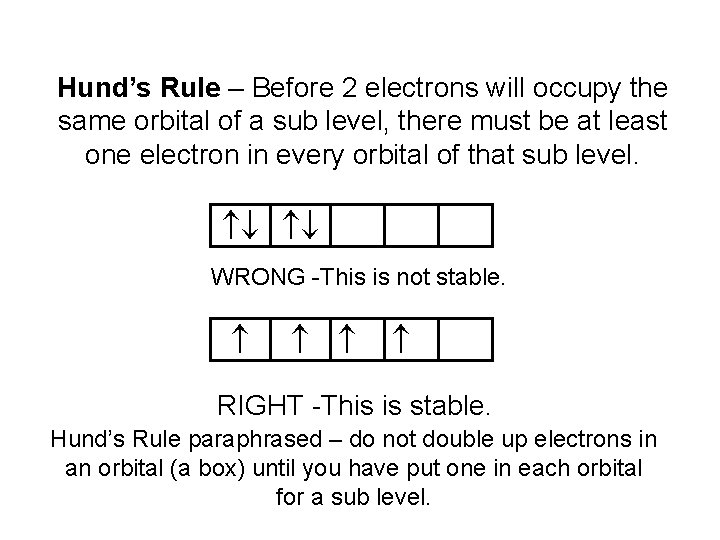
Hund’s Rule – Before 2 electrons will occupy the same orbital of a sub level, there must be at least one electron in every orbital of that sub level. WRONG -This is not stable. RIGHT -This is stable. Hund’s Rule paraphrased – do not double up electrons in an orbital (a box) until you have put one in each orbital for a sub level.

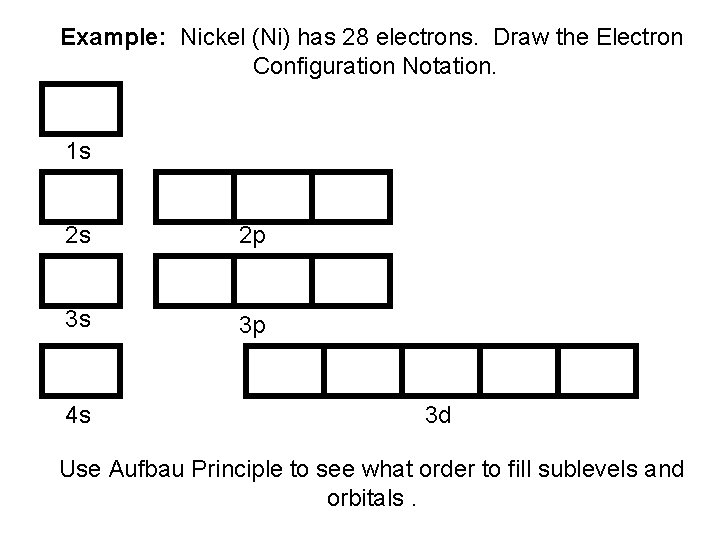
Example: Nickel (Ni) has 28 electrons. Draw the Electron Configuration Notation. 1 s 2 s 2 p 3 s 3 p 4 s 3 d Use Aufbau Principle to see what order to fill sublevels and orbitals.

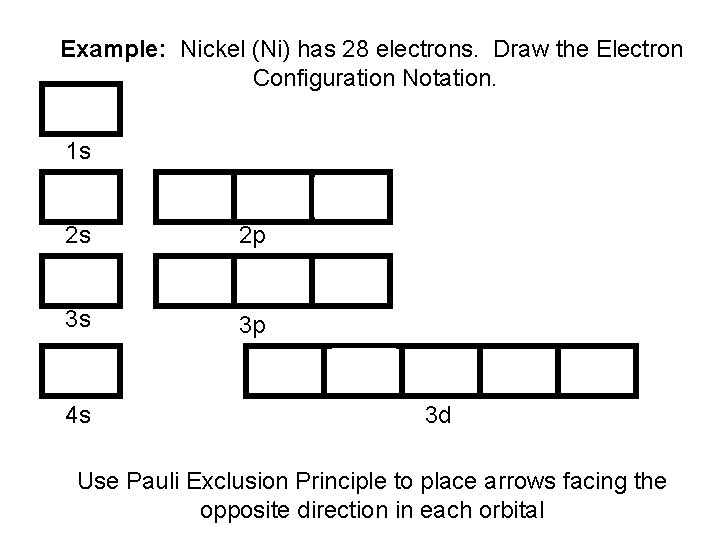
Example: Nickel (Ni) has 28 electrons. Draw the Electron Configuration Notation. 1 s 2 s 3 s 4 s 2 p 3 p 3 d Use Pauli Exclusion Principle to place arrows facing the opposite direction in each orbital

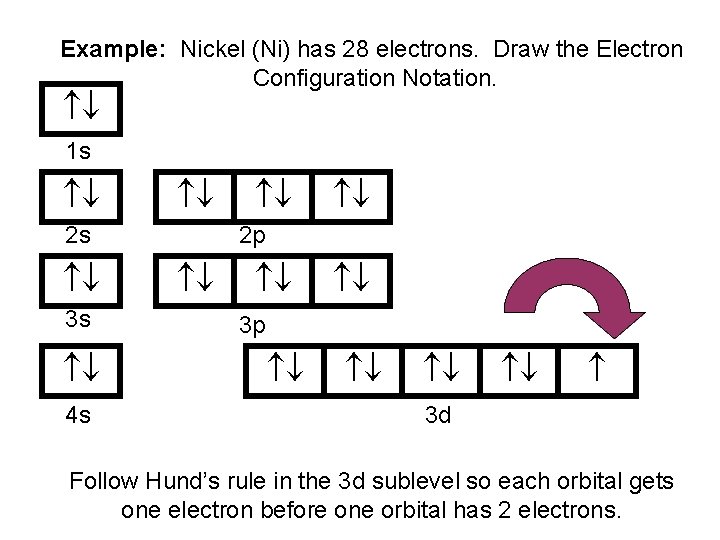
Example: Nickel (Ni) has 28 electrons. Draw the Electron Configuration Notation. 1 s 2 s 3 s 4 s 2 p 3 p 3 d Follow Hund’s rule in the 3 d sublevel so each orbital gets one electron before one orbital has 2 electrons.

Lewis Dot Structures Aka Dot Diagrams Electron Dot Diagrams

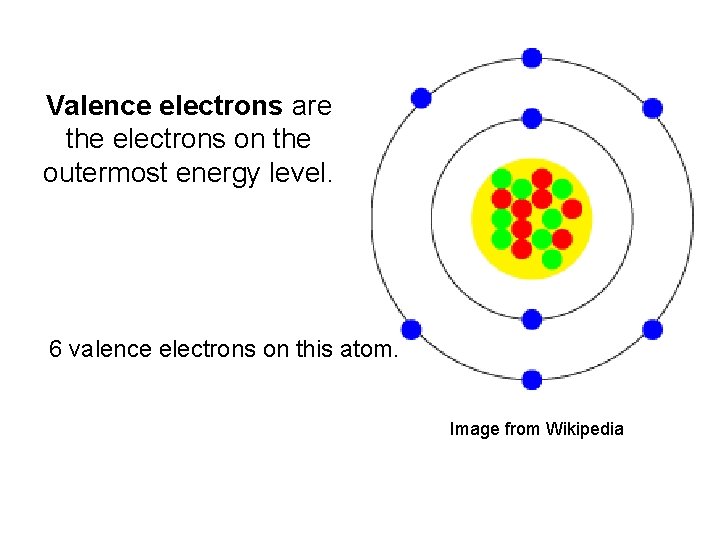
Valence electrons are the electrons on the outermost energy level. 6 valence electrons on this atom. Image from Wikipedia

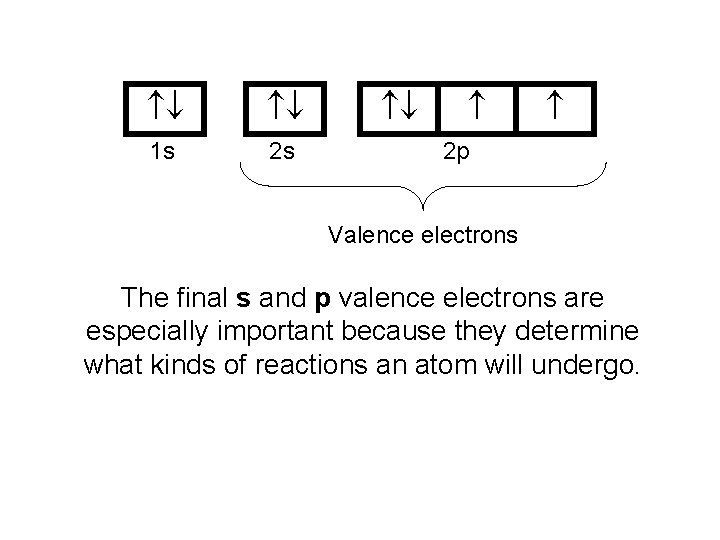
1 s 2 s 2 p Valence electrons The final s and p valence electrons are especially important because they determine what kinds of reactions an atom will undergo.

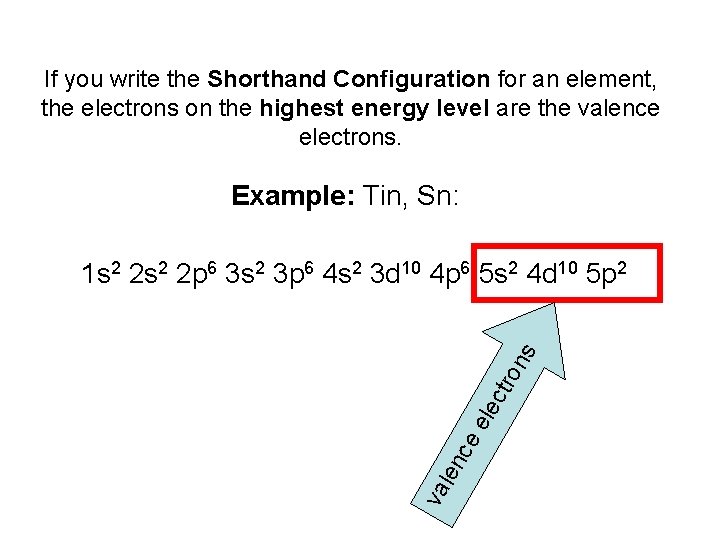
If you write the Shorthand Configuration for an element, the electrons on the highest energy level are the valence electrons. Example: Tin, Sn: va len ce ele ctr o ns 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 2

Lewis Dot Structures • A Lewis Dot Structure is a way of showing only the Valence electrons for an element. • This is very useful when showing how atoms react with one another. • A Lewis Dot Diagram consists of two parts: – the element symbol – dots that represent the S and P block electrons

Rules for Writing Lewis Dot Diagrams: • Dots may only go on the sides of the element symbol • Only use s and p valence electrons • If there are d or f valence electrons, they are left out of the dot diagram. • You must put 1 electron dot on each side of the element symbol before you put 2 on one side (like Hund’s rule)

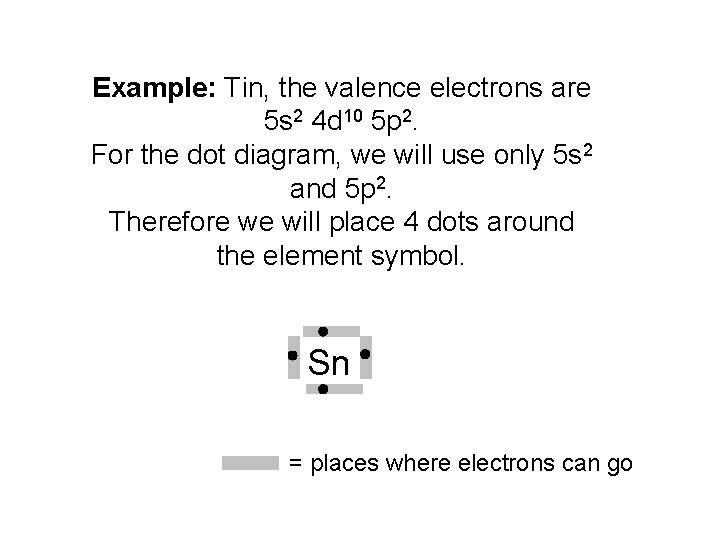
Example: Tin, the valence electrons are 5 s 2 4 d 10 5 p 2. For the dot diagram, we will use only 5 s 2 and 5 p 2. Therefore we will place 4 dots around the element symbol. Sn = places where electrons can go

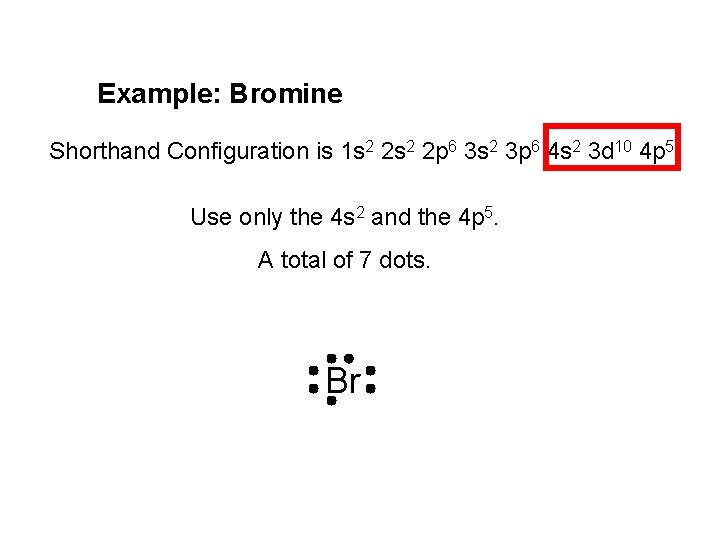
Example: Bromine Shorthand Configuration is 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5. Use only the 4 s 2 and the 4 p 5. A total of 7 dots. Br

Practice problems • Sodium • Neon • Aluminum

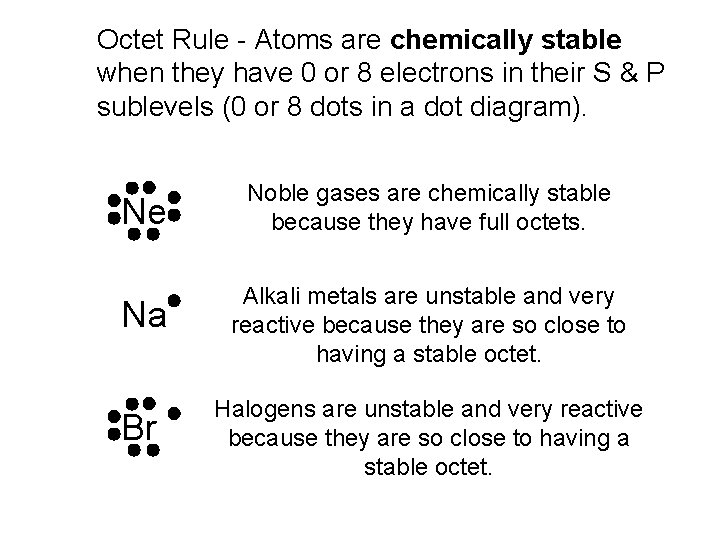
Octet Rule - Atoms are chemically stable when they have 0 or 8 electrons in their S & P sublevels (0 or 8 dots in a dot diagram). Ne Noble gases are chemically stable because they have full octets. Na Alkali metals are unstable and very reactive because they are so close to having a stable octet. Br Halogens are unstable and very reactive because they are so close to having a stable octet.

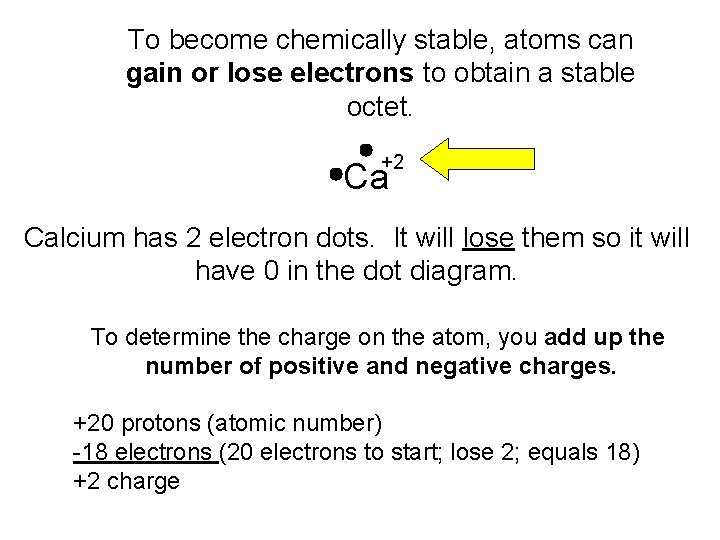
To become chemically stable, atoms can gain or lose electrons to obtain a stable octet. +2 Ca Calcium has 2 electron dots. It will lose them so it will have 0 in the dot diagram. To determine the charge on the atom, you add up the number of positive and negative charges. +20 protons (atomic number) -18 electrons (20 electrons to start; lose 2; equals 18) +2 charge

Ionization Rules • If an atom has 1, 2, or 3 electrons in a dot diagram, it will lose those electrons. • If an atom has 5, 6, or 7 electrons in a dot diagram, it will gain more to make 8. • If an atom has 4 electrons in a dot diagram, it can either gain or lose 4.

Alternatively, there are two rules you can memorize: • If atom loses electrons, it becomes positive. • If atom gains electrons, it becomes negative. Example: If it gains 1 electron, the atom becomes -1. If it loses 2 electrons, the atom becomes +2.

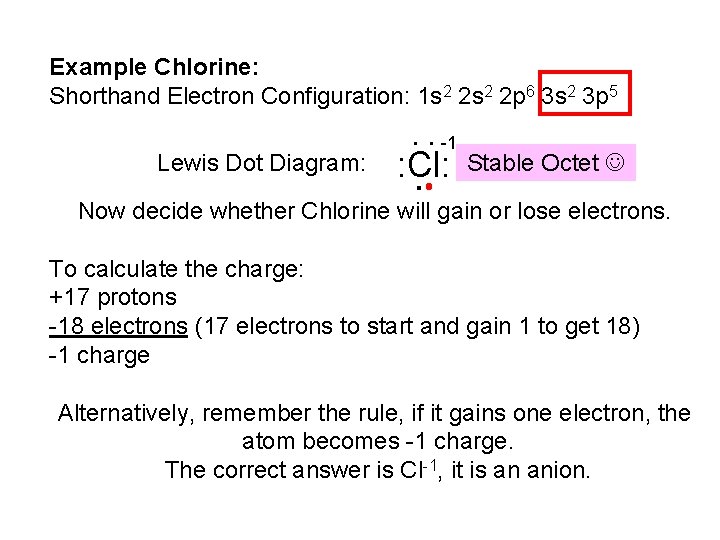
Example Chlorine: Shorthand Electron Configuration: 1 s 2 2 p 6 3 s 2 3 p 5 : -1 Lewis Dot Diagram: Stable Octet . : Cl: Now decide whether Chlorine will gain or lose electrons. To calculate the charge: +17 protons -18 electrons (17 electrons to start and gain 1 to get 18) -1 charge Alternatively, remember the rule, if it gains one electron, the atom becomes -1 charge. The correct answer is Cl-1, it is an anion.

Assignment • Complete the Dot Diagram Periodic Table Assignment. • Answer all questions on the back. • Be sure to label your Dot Diagram Periodic Table when the instructions say to. • Turn in the answers and the periodic table when you finish