Chemical Reactions Outline Chemical Reaction Chemical Equation Parts




- Slides: 13

Chemical Reactions

Outline Chemical Reaction Chemical Equation Parts of a chemical equation Skeleton Equation Types of Reactions

Chemical Reaction Process that leads to the transformation of one set of chemical substances to another.

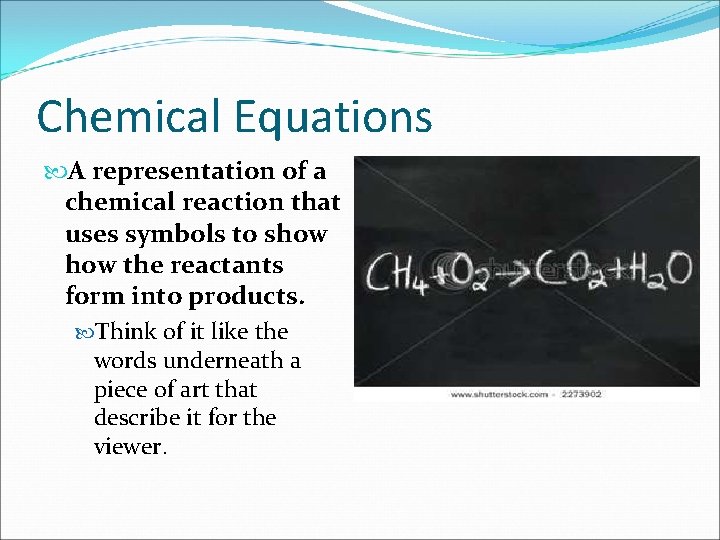
Chemical Equations A representation of a chemical reaction that uses symbols to show the reactants form into products. Think of it like the words underneath a piece of art that describe it for the viewer.

Parts of a chemical equation Reactants – Chemicals on the left side of the equation Products – Chemicals on the right side of the equation Reactants “react” to “produce” the products.

Skeleton Equations Chemical Equation that does not indicate the relative amounts of reactants and products I. E. This just lets us know what reactants and products are involved, not the correct amounts of them

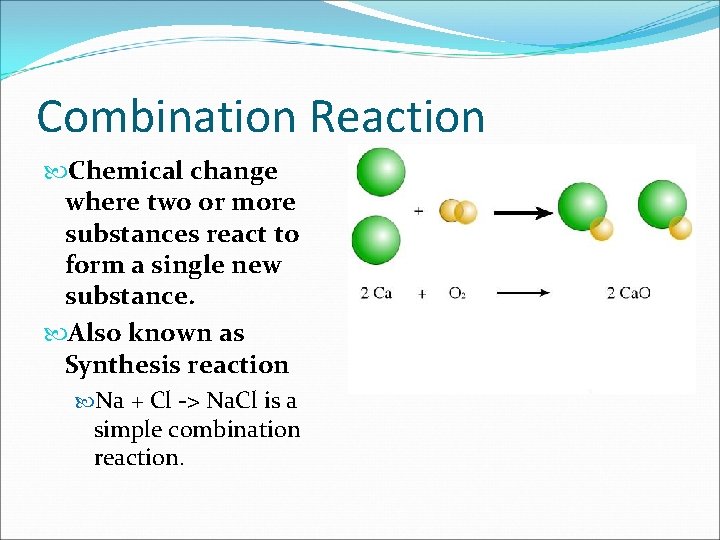
Combination Reaction Chemical change where two or more substances react to form a single new substance. Also known as Synthesis reaction Na + Cl -> Na. Cl is a simple combination reaction.

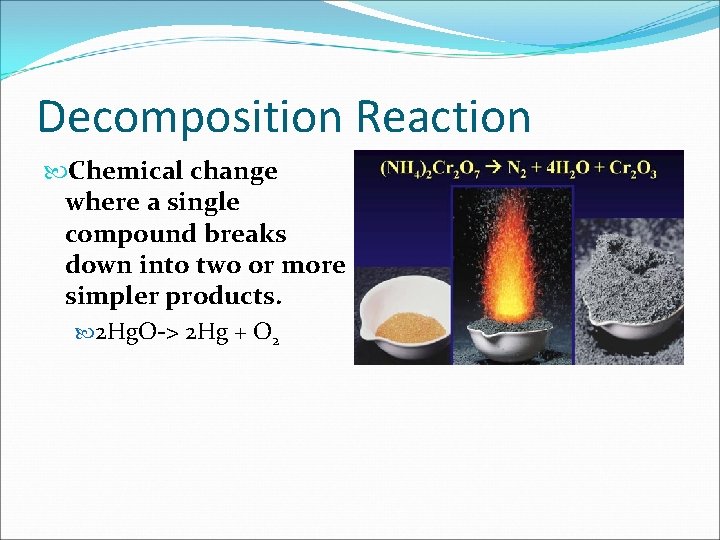
Decomposition Reaction Chemical change where a single compound breaks down into two or more simpler products. 2 Hg. O-> 2 Hg + O 2

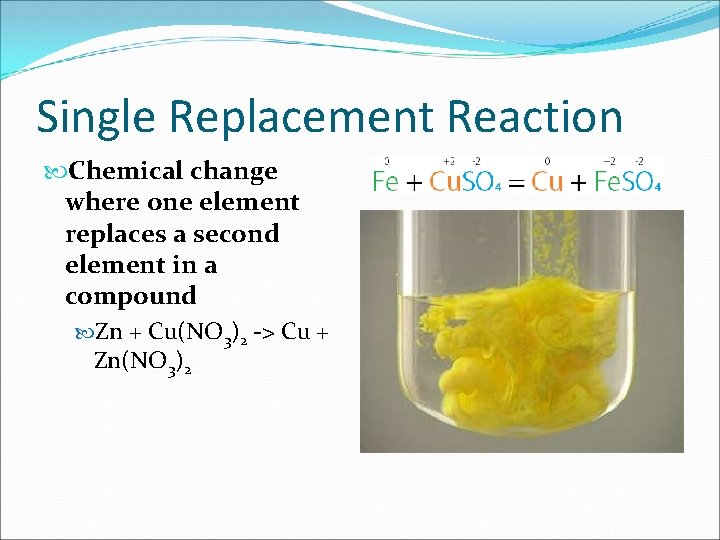
Single Replacement Reaction Chemical change where one element replaces a second element in a compound Zn + Cu(NO 3)2 -> Cu + Zn(NO 3)2

Activity Series Lists metals in decreasing order of reactivity. A metal will replace any metal beneath it on the chart. For example: Tin would replace Copper, but not Carbon.

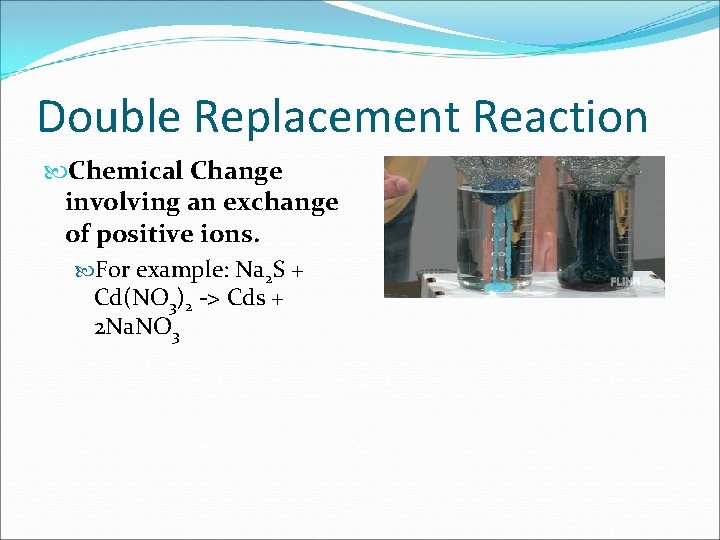
Double Replacement Reaction Chemical Change involving an exchange of positive ions. For example: Na 2 S + Cd(NO 3)2 -> Cds + 2 Na. NO 3

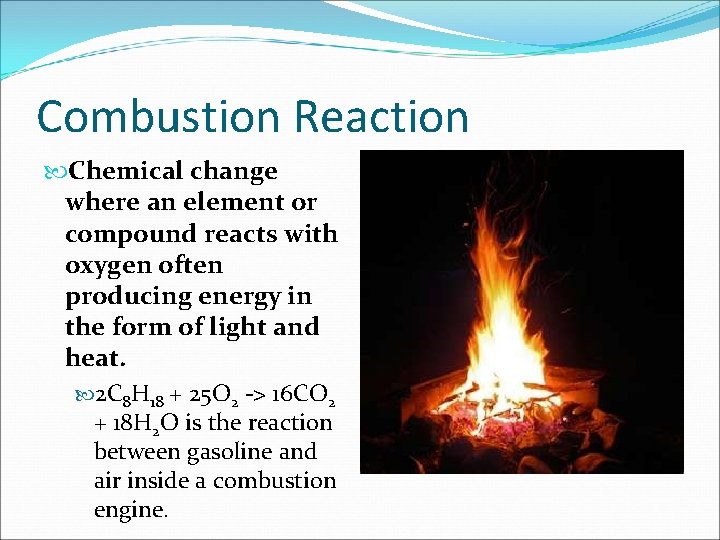
Combustion Reaction Chemical change where an element or compound reacts with oxygen often producing energy in the form of light and heat. 2 C 8 H 18 + 25 O 2 -> 16 CO 2 + 18 H 2 O is the reaction between gasoline and air inside a combustion engine.

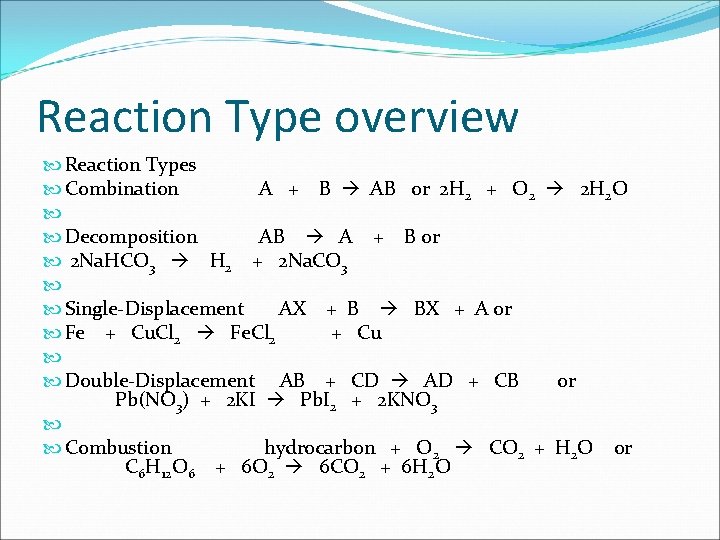
Reaction Type overview Reaction Types Combination A + B AB or 2 H 2 + O 2 2 H 2 O Decomposition AB A + B or 2 Na. HCO 3 H 2 + 2 Na. CO 3 Single-Displacement AX + B BX + A or Fe + Cu. Cl 2 Fe. Cl 2 + Cu Double-Displacement AB + CD AD + CB or Pb(NO 3) + 2 KI Pb. I 2 + 2 KNO 3 Combustion hydrocarbon + O 2 CO 2 + H 2 O or C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Examples of redox reaction
Examples of redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Synthesis reaction chemical equation
Synthesis reaction chemical equation Please excuse coughs sneezes and burps
Please excuse coughs sneezes and burps Quotation sandwhich
Quotation sandwhich Rate reaction equation
Rate reaction equation Ictahedron
Ictahedron Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction