The 65 th International Symposium on Molecular Spectroscopy






- Slides: 19

The 65 th International Symposium on Molecular Spectroscopy, June 2010 Optical Stark Spectroscopy of Iridium Monofluoride, Ir. F Xiujuan Zhuang & Timothy C. Steimle Dept. Chem. & Bio. Chem. Arizona State University, Tempe, AZ, USA Colan Linton Dept. Phys. & Chem, University of New Brunswick, Fredericton, NB, Canada Funded by: Do. E-BES

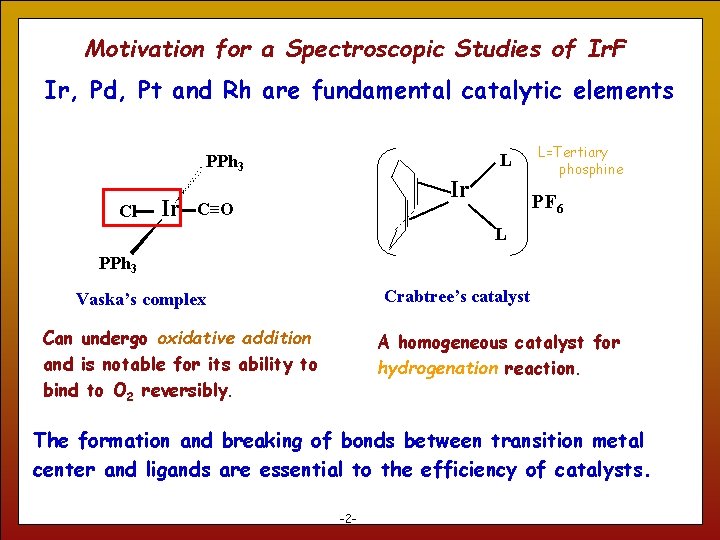
Motivation for a Spectroscopic Studies of Ir. F Ir, Pd, Pt and Rh are fundamental catalytic elements L PPh 3 Cl Ir Ir C≡O L=Tertiary phosphine PF 6 L PPh 3 Crabtree’s catalyst Vaska’s complex Can undergo oxidative addition and is notable for its ability to bind to O 2 reversibly. A homogeneous catalyst for hydrogenation reaction. The formation and breaking of bonds between transition metal center and ligands are essential to the efficiency of catalysts. 2 -2 -

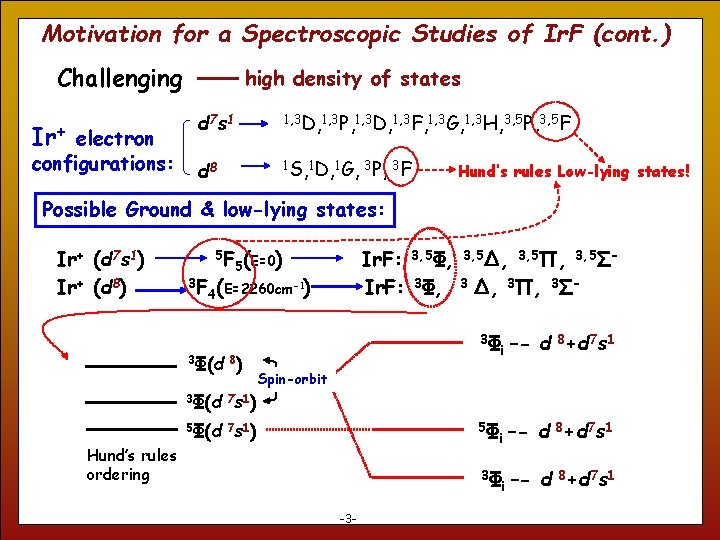
Motivation for a Spectroscopic Studies of Ir. F (cont. ) Challenging Ir+ electron configurations: high density of states d 7 s 1 1, 3 D, 1, 3 P, 1, 3 D, 1, 3 F, 1, 3 G, 1, 3 H, 3, 5 P, 3, 5 F d 8 1 S, 1 D, 1 G, 3 P, 3 F Hund’s rules Low-lying states! Possible Ground & low-lying states: Ir+ (d 7 s 1) Ir+ (d 8) 3 F 5 F 5(E=0) 4(E=2260 cm 3Φ(d 8) Ir. F: 3, 5Φ, 3, 5Δ, 3, 5Π, 3, 5ΣIr. F: 3Φ, 3 Δ, 3Π, 3Σ- ) -1 3Φ i –- d 8+d 7 s 1 3Φ –- d 8+d 7 s 1 Spin-orbit 3Φ(d 7 s 1) 5Φ 5Φ(d 7 s 1) Hund’s rules ordering 3 -3 - i

Previous Related Work – • Ir. F not much UNB group: Visible LIF and DF in a free-jet expansion Fine structure parameters for numerous visible bands “red system” A 3Φ 4 -X 3Φ 4 Stark spectrum of Ir. F A 3Φ 4 -X 3Φ 4 No theoretical calculations! Today's talk • Ir-related molecules: Ir. C & Ir. N ASU group: Magnetic hyperfine interactions Permanent electric dipole J. Chem. Phys. 104, 8183, 1996 The goal: Determine μ for Ir. F, and compare with Ir. C, Ir. N and Co. F 4 Fe 26 Co 27 Ni 28 [Ar]3 d 64 s 2 [Ar]3 d 74 s 2 [Ar]3 d 84 s 2 Ru 44 Rh 45 Pd 46 [Kr]4 d 75 s 1 [Kr]4 d 85 s 1 [Kr]4 d 10 Os 76 Ir 77 Pt 78 [Xe]4 f 145 d 66 s 2 [Xe]4 f 145 d 76 s 2 [Xe]4 f 145 d 96 s 1 Extensive studies on isovalent Co. F -4 -

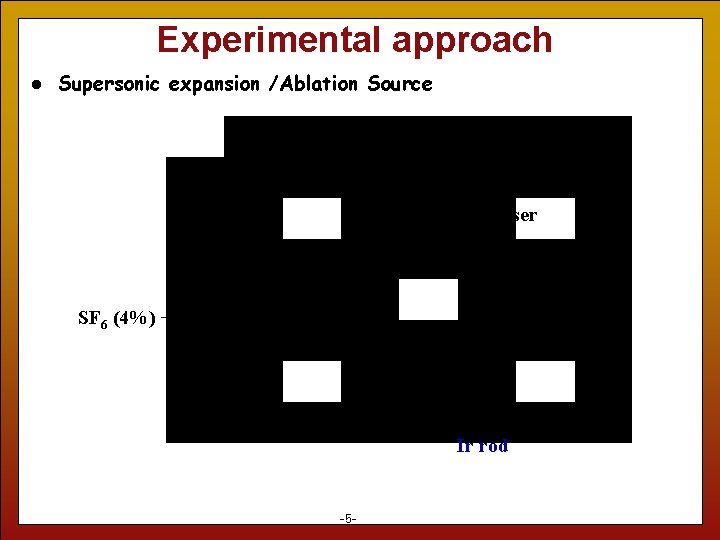
Experimental approach l Supersonic expansion /Ablation Source 532 nm Nd: YAG Pulse Laser Pulse Valve SF 6 (4%) + Ar Plasma Ir rod 5 -5 -

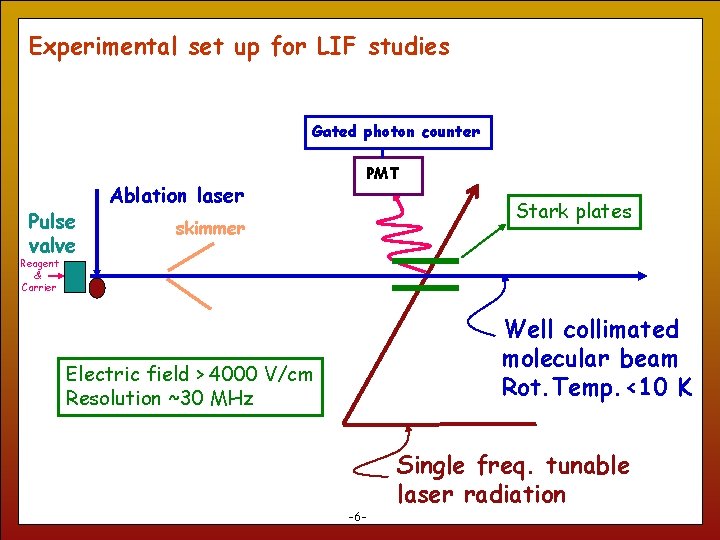
Experimental set up for LIF studies Gated photon counter Pulse valve Ablation laser PMT Stark plates skimmer Reagent & Carrier Well collimated molecular beam Rot. Temp. <10 K Electric field > 4000 V/cm Resolution ~30 MHz 6 -6 - Single freq. tunable laser radiation

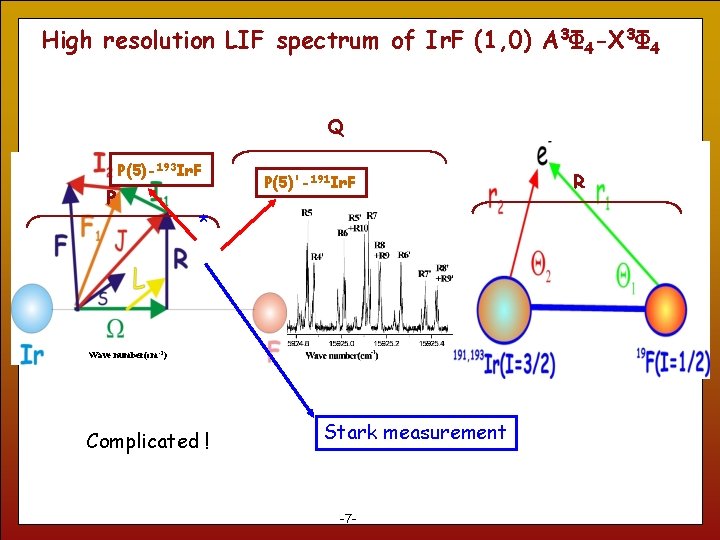
High resolution LIF spectrum of Ir. F (1, 0) A 3Φ 4 -X 3Φ 4 Q P(5)-193 Ir. F P P(5)'-191 Ir. F * Wave number(cm-1) Complicated ! 7 Stark measurement -7 - R

Why are the spectrum complicated ? 1) Two nuclear spin open shell molecule: Atom Abundance (%) g. N I Mag. (m. N ) e. Q. (Barnes) 191 Ir 37 0. 101 3/2 0. 151 0. 82 193 Ir 63 0. 109 3/2 0. 164 0. 75 19 F 100 5. 376 1/2 2. 629 _ 2) The 19 F magnetic hyperfine interaction is comparable to the F 1 is not an approximately good quantum number. F 1 J=4 X 3Φ 4 A 3Φ 4 8 Rotation 191, 193 Ir magnetic hyperfine. F 6 5 5. 5 4. 5 5 4 3 2. 5 3 2 2. 5 Mag. (Ir) [Mag. +Quad. ] (Ir) -8 - hyf(Ir)+hyf(F)

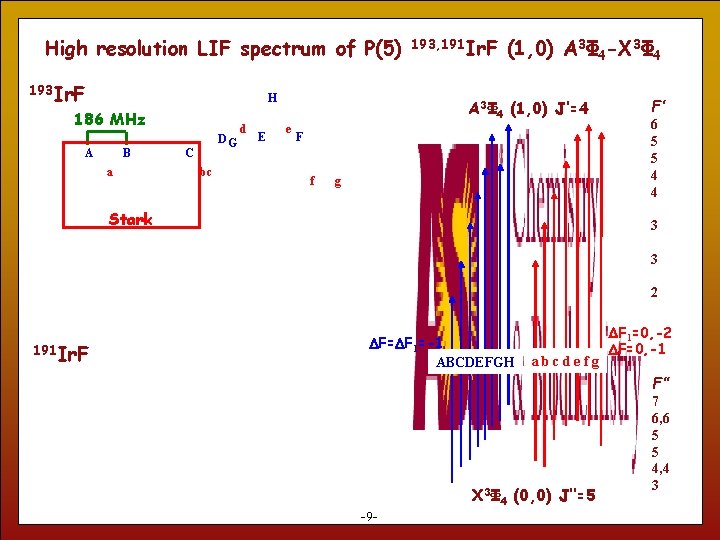
High resolution LIF spectrum of P(5) 193 Ir. F H 186 MHz A B a DG C bc d E 193, 191 Ir. F (1, 0) A 3Φ 4 -X 3Φ 4 A 3Φ 4 (1, 0) J’=4 e F f g Stark F’ 6 5 5 4 4 3 3 2 191 Ir. F DF=DF 1=-1 ABCDEFGH abcdefg DF 1=0, -2 DF=0, -1 F’’ 9 -9 - X 3Φ 4 (0, 0) J’’=5 7 6, 6 5 5 4, 4 3

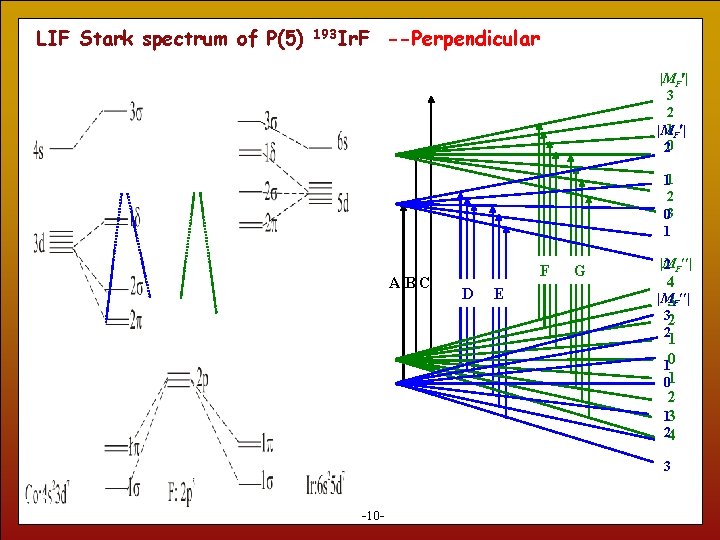
LIF Stark spectrum of P(5) 193 Ir. F --Perpendicular |MF | 3 2 |M 1 F | 20 11 2 03 1 ABC F D E G |M 2 F | 4 |M 3 F | 32 2 1 10 01 2 13 24 3 10 -10 -

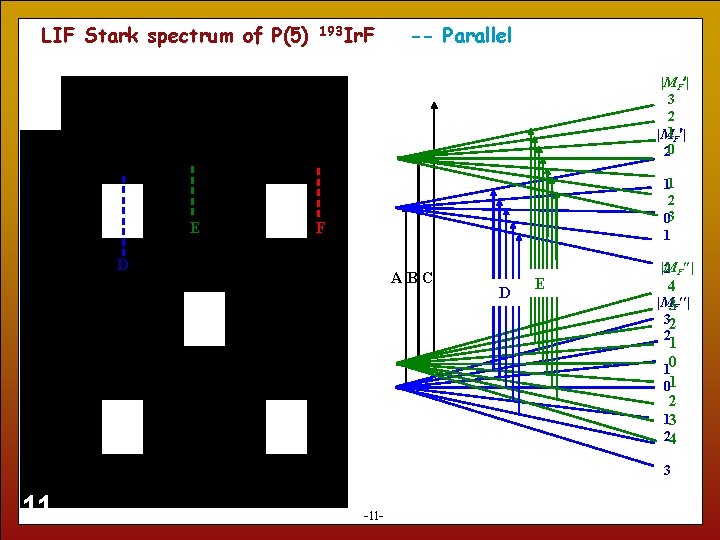
LIF Stark spectrum of P(5) 193 Ir. F -- Parallel |MF | 3 2 |M 1 F | 20 E 11 2 03 1 F D ABC D E |M 2 F | 4 |M 3 F | 32 2 1 10 01 2 13 24 3 11 -11 -

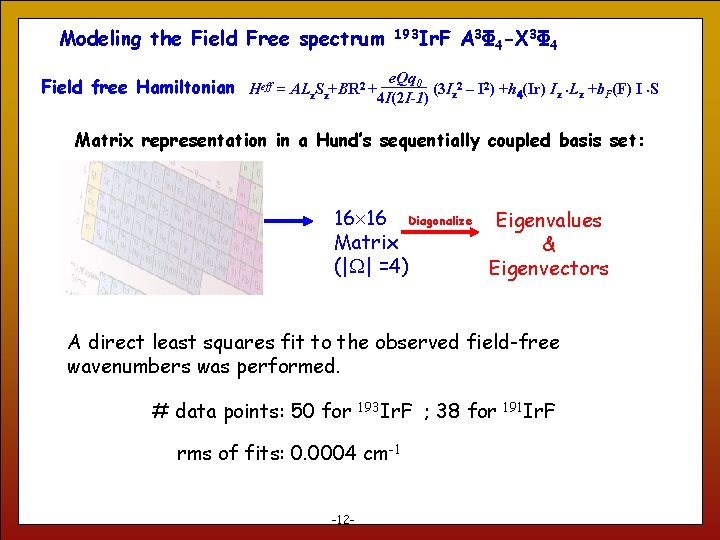
Modeling the Field Free spectrum 193 Ir. F A 3Φ 4 -X 3Φ 4 e. Qq 0 Field free Hamiltonian Heff = ALz. Sz+BR 2 + (3 I 2 – I 2) +h 4(Ir) Iz Lz +b. F(F) I S 4 I(2 I-1) z Matrix representation in a Hund’s sequentially coupled basis set: 16 16 Diagonalize Eigenvalues Matrix & (| | =4) Eigenvectors A direct least squares fit to the observed field-free wavenumbers was performed. # data points: 50 for 193 Ir. F rms of fits: 0. 0004 cm-1 12 -12 - ; 38 for 191 Ir. F

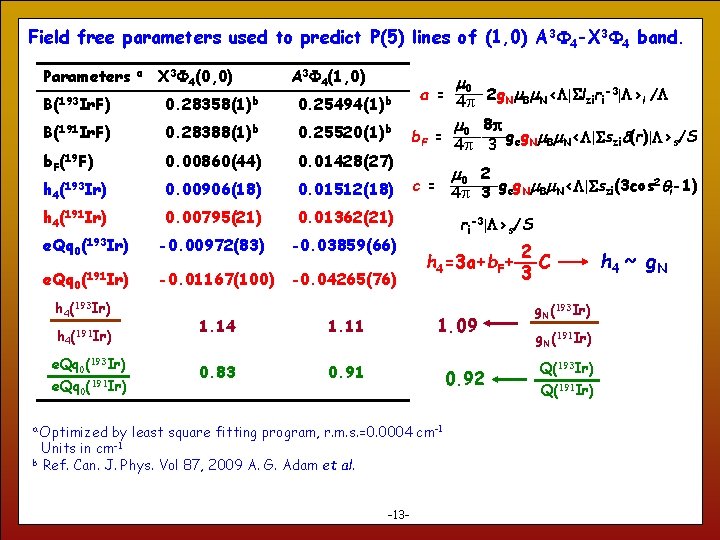
Field free parameters used to predict P(5) lines of (1, 0) A 3 4 -X 3 4 band. Parameters a X 3 4(0, 0) A 3 4(1, 0) m B(193 Ir. F) 0. 28358(1)b 0. 25494(1)b B(191 Ir. F) 0. 28388(1)b 0. 25520(1)b b. F(19 F) 0. 00860(44) 0. 01428(27) (193 Ir) 0. 00906(18) 0. 01512(18) h 4(191 Ir) 0. 00795(21) 0. 01362(21) -0. 00972(83) -0. 03859(66) -0. 01167(100) -0. 04265(76) h 4 e. Qq 0(193 Ir) e. Qq 0 (191 Ir) h 4(193 Ir) h 4(191 Ir) e. Qq 0(193 Ir) e. Qq 0(191 Ir) 1. 14 1. 11 0. 83 0. 91 0 a = 4 p 2 g. Nm. Bm. N<L|Slziri-3|L>l /L m 0 8 p b. F = 4 p 3 geg. Nm. Bm. N<L|Sszid(r)|L>s/S m 0 2 2 c = 4 p 3 geg. Nm. Bm. N<L|Sszi(3 cos qi-1) ri-3|L>s/S h 4=3 a+b. F+ 1. 09 0. 92 Optimized by least square fitting program, r. m. s. =0. 0004 cm-1 Units in cm-1 b Ref. Can. J. Phys. Vol 87, 2009 A. G. Adam et al. a 13 -13 - 2 C 3 g. N(193 Ir) g. N(191 Ir) Q(193 Ir) Q(191 Ir) h 4 ~ g. N

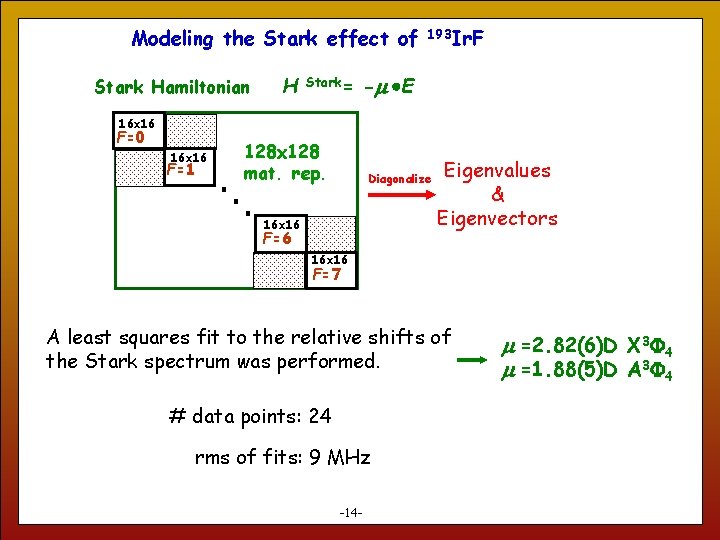
Modeling the Stark effect of Stark Hamiltonian H Stark= 193 Ir. F -m E 16 x 16 F=0 16 x 16 F=1 128 x 128 mat. rep. Diagonalize 16 x 16 F=6 Eigenvalues & Eigenvectors 16 x 16 F=7 A least squares fit to the relative shifts of the Stark spectrum was performed. # data points: 24 rms of fits: 9 MHz 14 -14 - m =2. 82(6)D X 3 4 m =1. 88(5)D A 3 4

Comparison of ground state dipole moments a b Molecules Ir. C Ir. N Ir. F Co. F m (D) 1. 60(7) 1. 66(1) 2. 82(6) 4. 51(5) state X 2 D 5/2 X 1 S + X 3 4 Re(Å) 1. 6086 1. 6094 1. 8507 b 1. 7358 m/Re 0. 99 1. 03 1. 52 2. 60 All the m and Re come from experimental measurements of Prof. Tim Steimle’s group Ref. Can. J. Phys. Vol 87, 2009 A. G. Adam et al. Electronegativity: (in Pauling scale) Ir F Ir N 2. 20 3. 98 2. 20 3. 04 Ir C 2. 20 2. 55 Co 1. 88 15 a F 3. 98 Consistent with electronegativity ! -15 -

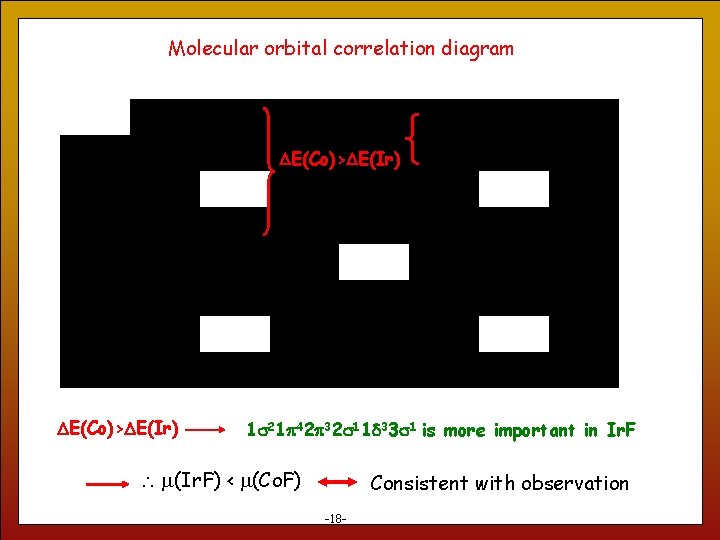
Molecular orbital correlation diagram Small due to relativistic stabilization 1 s 221 p 1 p 442 p 2 p 332 s 2 s 221 d 1 d 33 i 1 s 21 p 42 p 32 s 11 d 33 s 1 3 i 16 -16 -

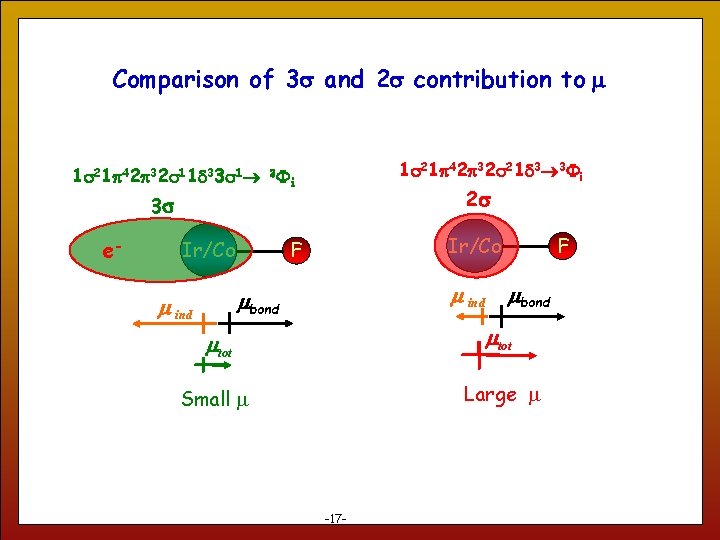
Comparison of 3 s and 2 s contribution to m 1 s 21 p 42 p 32 s 21 d 3 3 i 1 s 21 p 42 p 32 s 11 d 33 s 1 3 i 2 s 3 s e- Ir/Co m ind mbond m ind 17 Ir/Co F F mbond mtot Small m Large m -17 -

Molecular orbital correlation diagram DE(Co)>DE(Ir) 18 1 s 21 p 42 p 32 s 11 d 33 s 1 is more important in Ir. F m(Ir. F) < m(Co. F) Consistent with observation -18 -

Conclusion a) The complicated hyperfine structure of low-J lines has been analyzed. b) The permanent electric dipole moment were obtained form optical Stark spectrum. Still to do: a) Interpret the hyperfine parameters. b) Theoretical predictions (by others). Thank you 19 -19 -
 International symposium on molecular spectroscopy
International symposium on molecular spectroscopy Erzeng
Erzeng Jpl molecular spectroscopy
Jpl molecular spectroscopy Applications of uv visible spectroscopy
Applications of uv visible spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium Chara screenshot
Chara screenshot International police executive symposium
International police executive symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Melting and boiling point of oxygen
Melting and boiling point of oxygen Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể độ dài liên kết
độ dài liên kết Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua