The 64 th International Symposium on Molecular Spectroscopy













- Slides: 31

The 64 th International Symposium on Molecular Spectroscopy, TF 15 Columbus, Ohio, June 22 -26, 2009 Spectroscopic identification of p-chloro-α -methylbenzyl radical in the gas phase Seung Woon Lee, Gi Woo Lee, Sang Kuk Lee Department of Chemistry Pusan National University Pusan 609 -735, Korea Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Previous works Mono-substituted benzyl radicals X = F, Cl, CH 3, CN Multi-substituted benzyl radicals X = F, Cl, CH 3 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

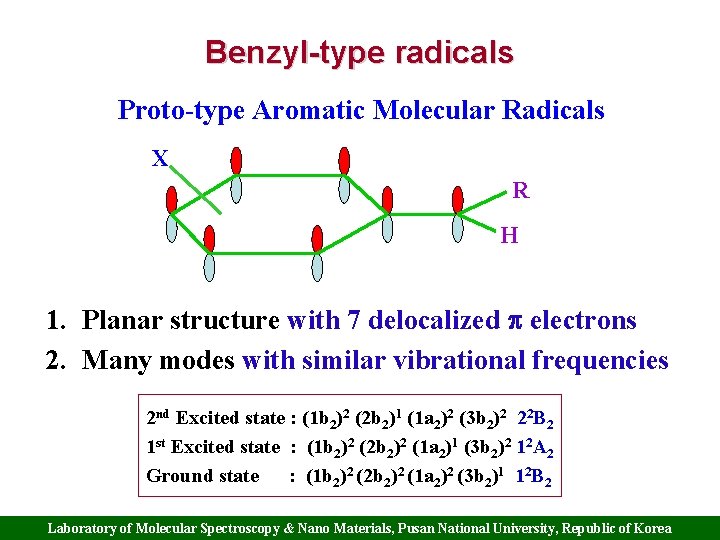
Benzyl-type radicals Proto-type Aromatic Molecular Radicals X R H 1. Planar structure with 7 delocalized electrons 2. Many modes with similar vibrational frequencies 2 nd Excited state : (1 b 2)2 (2 b 2)1 (1 a 2)2 (3 b 2)2 22 B 2 1 st Excited state : (1 b 2)2 (2 b 2)2 (1 a 2)1 (3 b 2)2 12 A 2 Ground state : (1 b 2)2 (2 b 2)2 (1 a 2)2 (3 b 2)1 12 B 2 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

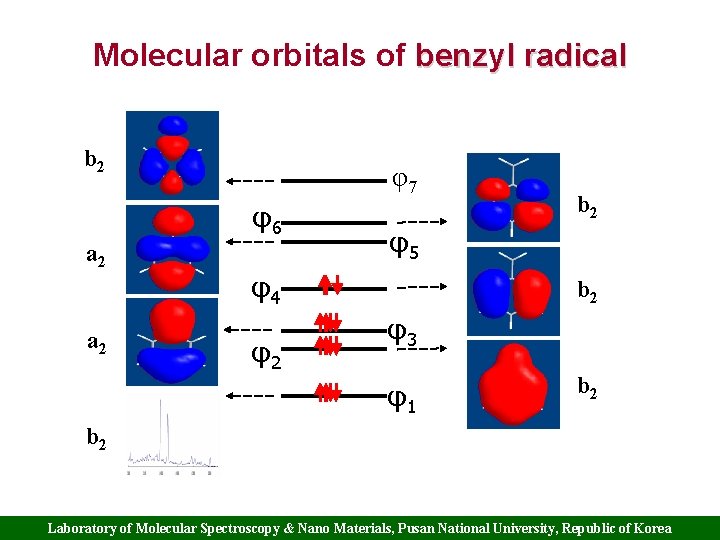
Molecular orbitals of benzyl radical b 2 φ7 φ6 a 2 φ5 φ4 φ2 b 2 φ3 φ1 b 2 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

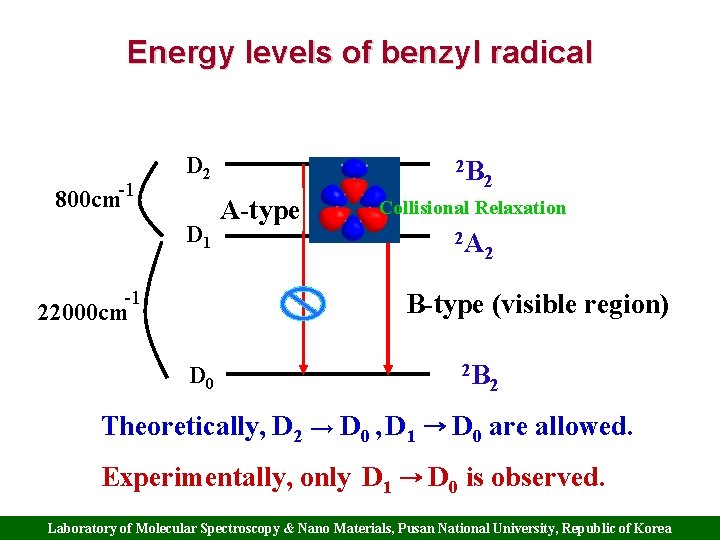
Energy levels of benzyl radical -1 D 2 800 cm D 1 -1 2 B A-type 2 Collisional Relaxation 2 A 2 B-type (visible region) 22000 cm D 0 2 B 2 Theoretically, D 2 → D 0 , D 1 → D 0 are allowed. Experimentally, only D 1 → D 0 is observed. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

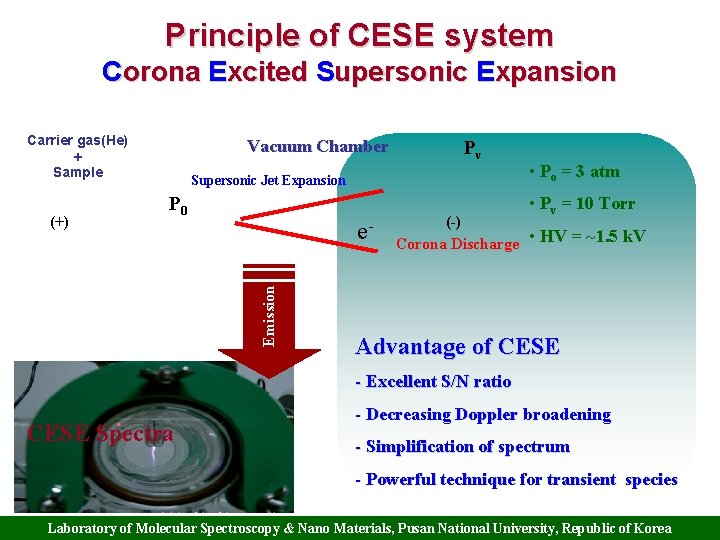
Principle of CESE system Corona Excited Supersonic Expansion Carrier gas(He) + Sample Pv Supersonic Jet Expansion P 0 • Po = 3 atm • Pv = 10 Torr e. Emission (+) Vacuum Chamber (-) Corona Discharge • HV = ~1. 5 k. V Advantage of CESE - Excellent S/N ratio CESE Spectra - Decreasing Doppler broadening - Simplification of spectrum - Powerful technique for transient species Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

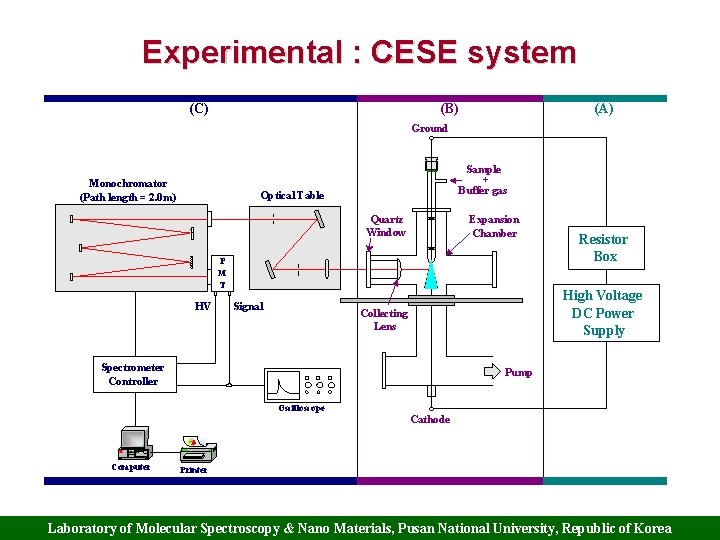
Experimental : CESE system (C) (B) (A) Ground Monochromator (Path length = 2. 0 m) Sample + Buffer gas Optical Table Quartz Window Expansion Chamber P M T HV Signal Resistor Box High Voltage DC Power Supply Collecting Lens Spectrometer Controller Pump Oscilloscope Cathode Computer Printer Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Overview of CESE system Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Jet emission in CESE system Discharge in CESE Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

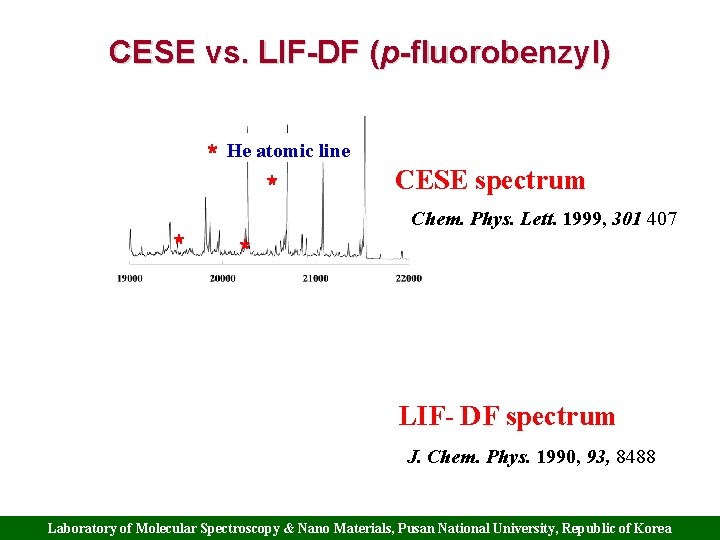
CESE vs. LIF-DF (p-fluorobenzyl) * He atomic line * * CESE spectrum Chem. Phys. Lett. 1999, 301 407 * LIF- DF spectrum J. Chem. Phys. 1990, 93, 8488 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

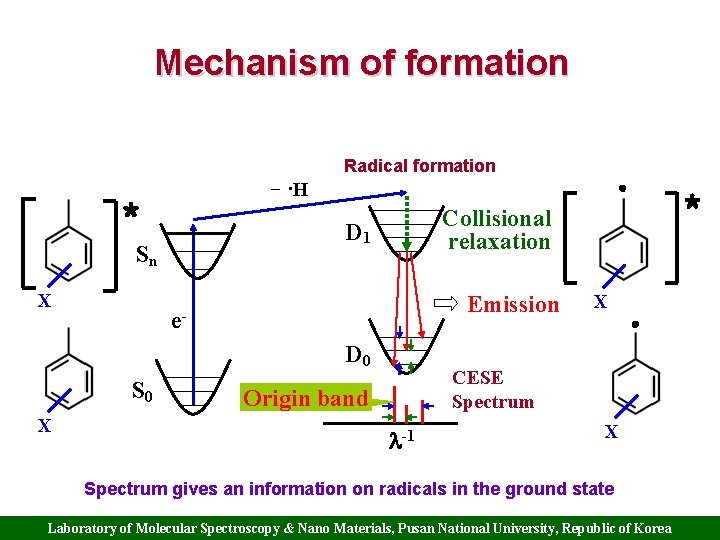
Mechanism of formation Radical formation − ·H * D 1 Sn X Emission e. D 0 S 0 X * Collisional relaxation X CESE Spectrum Origin band -1 X Spectrum gives an information on radicals in the ground state Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Radical from toluene Precursor : Toluene 6 b 6 a 1 Origin -H Do(C-H) = 415 k. J/mol Toluene Benzyl radical Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Radicals from ethylbenzene strong transition: benzyl radical Precursor: Ethylbenzene weak intensity: α-methylbenzyl radical(? ) Major product Do(C-C) = 344 k. J/mol Do(C-H) = 415 k. J/mol Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

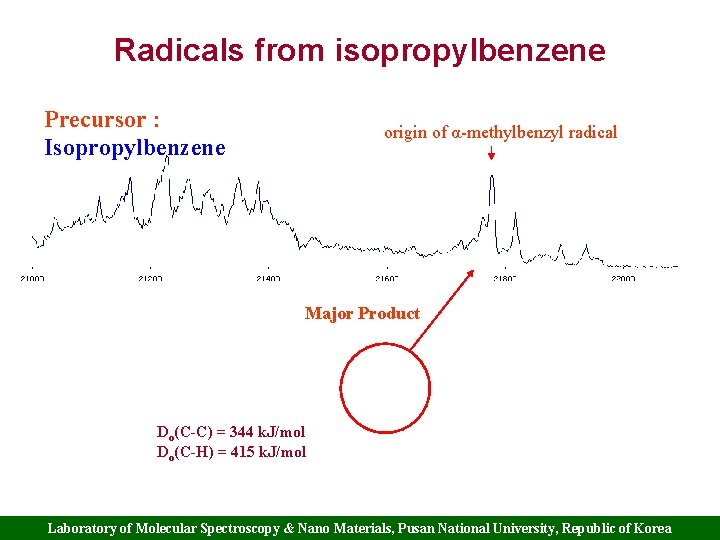
Radicals from isopropylbenzene Precursor : Isopropylbenzene origin of α-methylbenzyl radical Major Product Do(C-C) = 344 k. J/mol Do(C-H) = 415 k. J/mol Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

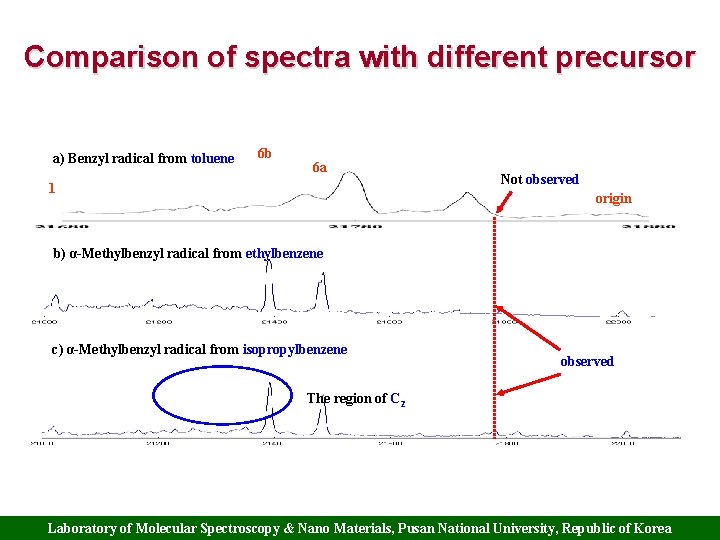
Comparison of spectra with different precursor a) Benzyl radical from toluene 6 b 6 a 1 Not observed origin b) α-Methylbenzyl radical from ethylbenzene c) α-Methylbenzyl radical from isopropylbenzene observed The region of C 2 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

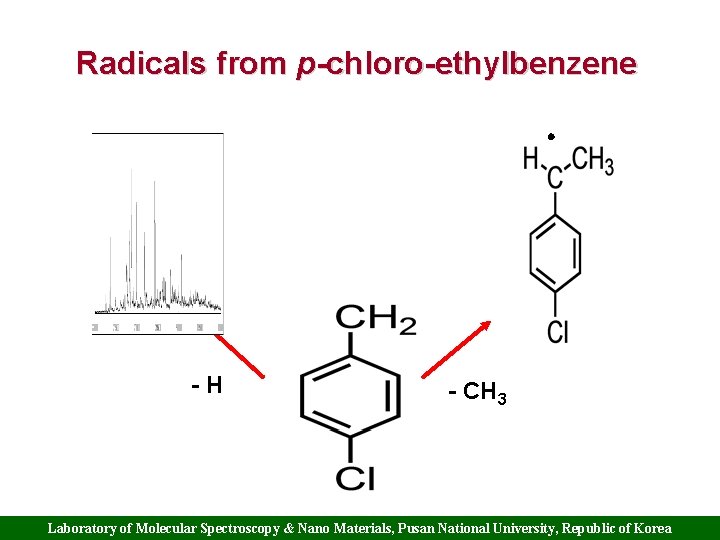
Radicals from p-chloro-ethylbenzene -H - CH 3 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

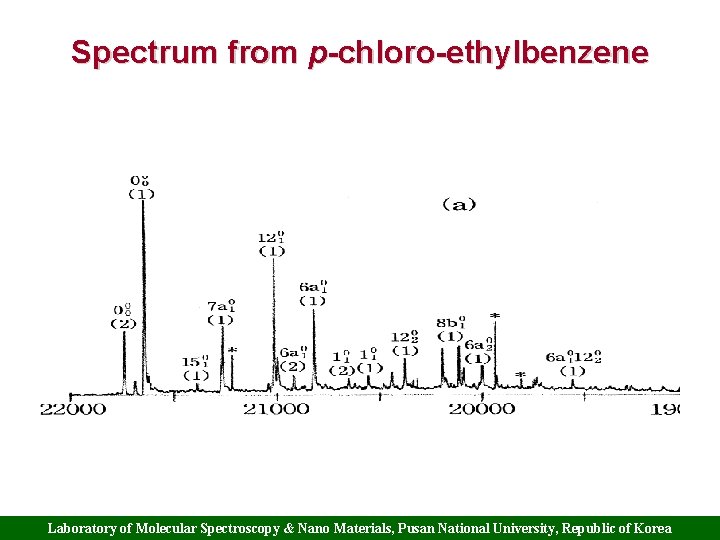
Spectrum from p-chloro-ethylbenzene Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

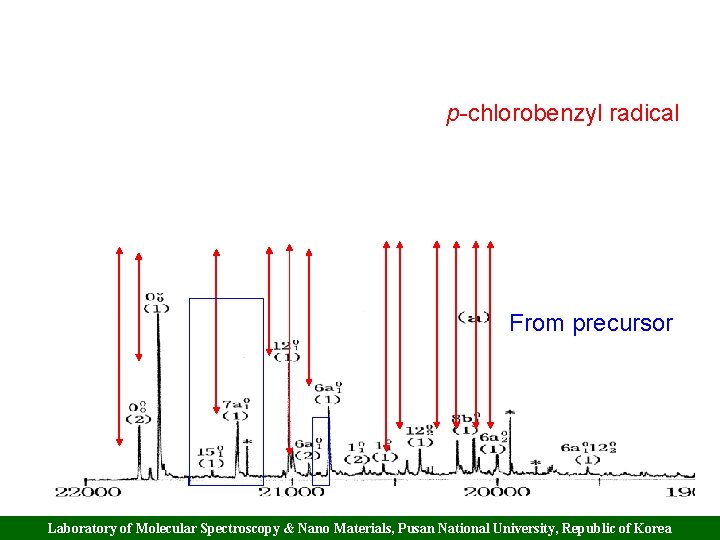
p-Chlorobenzyl radical Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

p-chlorobenzyl radical From precursor Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

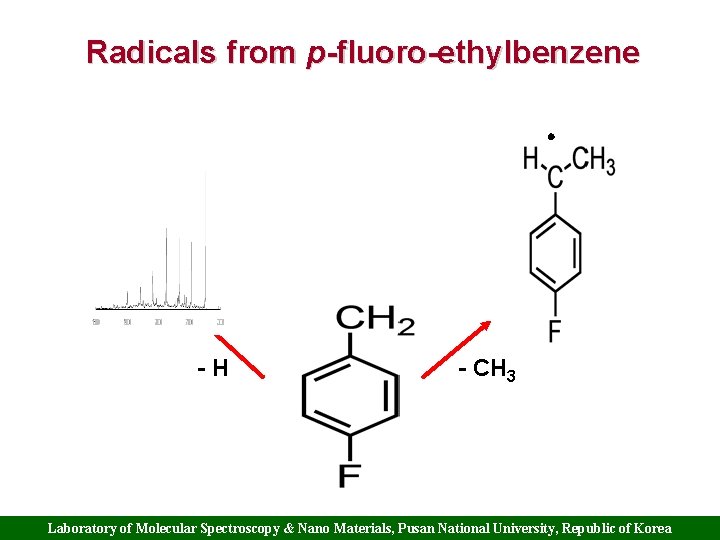
Radicals from p-fluoro-ethylbenzene -H - CH 3 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

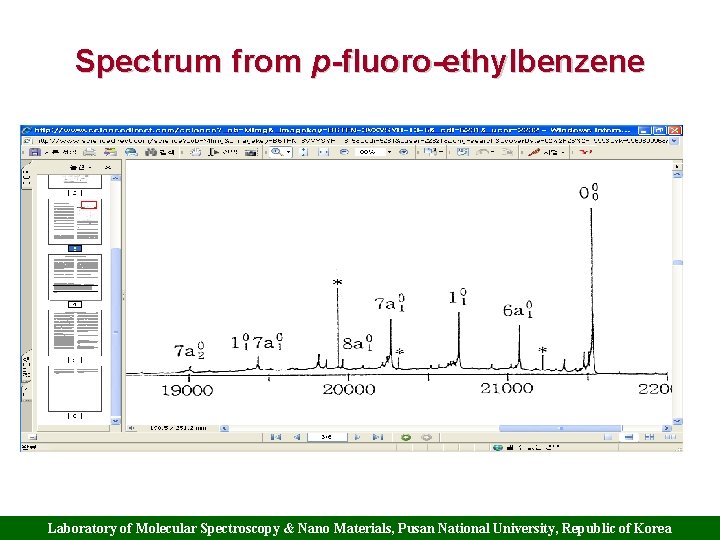
Spectrum from p-fluoro-ethylbenzene Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

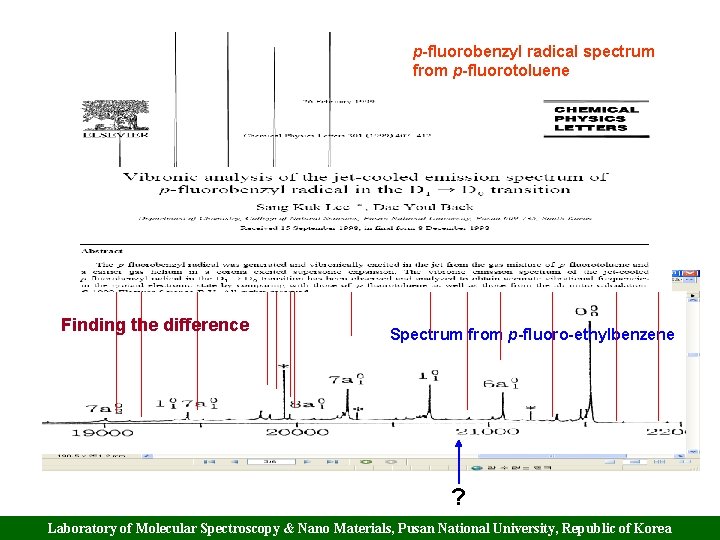
p-fluorobenzyl radical spectrum from p-fluorotoluene Finding the difference Spectrum from p-fluoro-ethylbenzene ? Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

The difference in spectra Origin 1 6 a Wavenumber (cm-1) Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

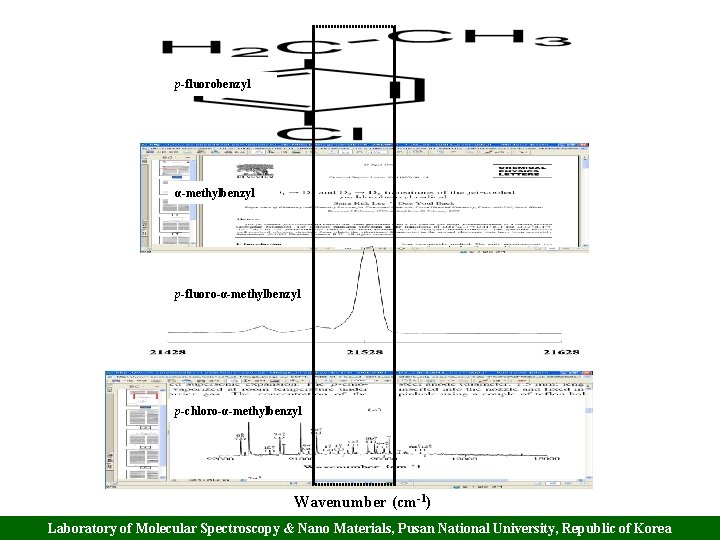
p-fluorobenzyl α-methylbenzyl p-fluoro-α-methylbenzyl p-chloro-α-methylbenzyl Wavenumber (cm-1) Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

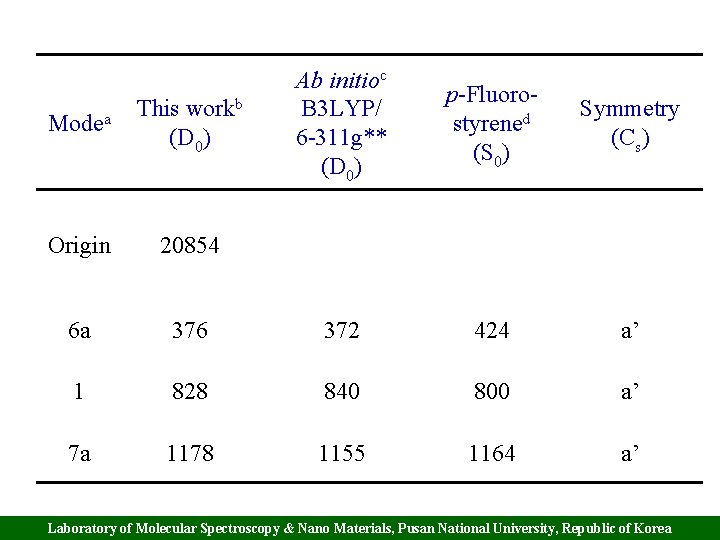
Modea workb This (D 0) Ab initioc B 3 LYP/ 6 -311 g** (D 0) p-Fluorostyrened (S 0) Symmetry (Cs) Origin 20854 6 a 376 372 424 a’ 1 828 840 800 a’ 7 a 1178 1155 1164 a’ Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Molecules benzylb o-fluorobenzylc m-fluorobenzylc p-fluorobenzylc 2, 3 -difluorobenzyle 2, 4 -difluorobenzyle 2, 5 -difluorobenzyle 2, 6 -difluorobenzyle 3, 4 -difluorobenzyle 3, 5 -difluorobenzylf α-methylbenzylg p-fluoro-α-methylbenzylh Origin band 22002 21924 21691 Shiftd 0 78 311 21527 21338 21846 21048 21774 21962 21182 21778 20854 475 664 156 954 228 40 820 224 1148 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

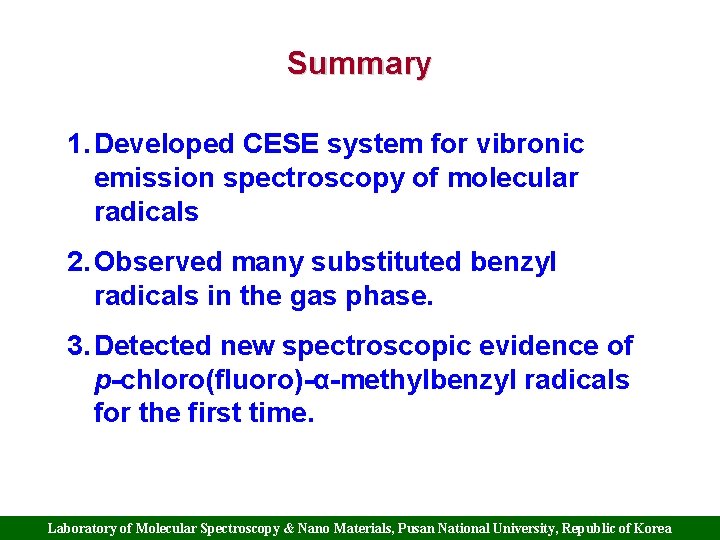
Summary 1. Developed CESE system for vibronic emission spectroscopy of molecular radicals 2. Observed many substituted benzyl radicals in the gas phase. 3. Detected new spectroscopic evidence of p-chloro(fluoro)-α-methylbenzyl radicals for the first time. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Acknowledgments Funding for Basic Sciences 1. Basic Research Program, Korea Research Foundation 2. Special Basic Research Program, Korea Science and Engineering Foundation Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea

Thank you for your attention Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
 International symposium on molecular spectroscopy
International symposium on molecular spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Jpl molecular spectroscopy
Jpl molecular spectroscopy Introduction to molecular spectroscopy
Introduction to molecular spectroscopy International police executive symposium
International police executive symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium Chara screenshot
Chara screenshot Physical state of covalent compounds
Physical state of covalent compounds Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Cái miệng nó xinh thế
Cái miệng nó xinh thế Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Ví dụ giọng cùng tên
Ví dụ giọng cùng tên Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Hát lên người ơi alleluia
Hát lên người ơi alleluia Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Diễn thế sinh thái là
Diễn thế sinh thái là đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính thế năng
Công thức tính thế năng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em