T 7 005 Survival and Prognostic Factors for


















- Slides: 19

T 7 -005 Survival and Prognostic Factors for Patients with Advanced Hepatocellular Carcinoma after Stereotactic Ablative Radiotherapy Wen-Yen Huang (黃文� ), Cheng-Hsiang Lo, Jen-Fu Yang, Ming-Yueh Liu, Yee-Min Jen, Chun-Shu Lin, Hsing-Lung Chao Department of Radiation Oncology Tri-Service General Hospital

DISCLOSURE OF CONFLICT OF INTEREST: NONE

RADIOTHERAPY IN LIVER TUMORS ¢ RT was not standard treatment for liver cancer. ¢ Limitation: * poor radiation tolerance of normal liver * organ motion





SABR FOR LIVER CANCER INTSGH ¢ Research overview 1. Indications (safety and efficacy) (1) Recurrent HCC (Int J Radiat Oncol Biol Phys 2012) (2) TACE-failed HCC (European Journal of Gastroenterology & Hepatology 2014) (3) Re-irradiation for HCC (Journal of Gastroenterology and Hepatology 2014) 2. Identify prognostic indicators (1) 18 F-FDG PET parameters (J Nucl Med 2013) 3. Image response evaluation (1) Radiographic response evaluation for PVTT (Therapeut Radiol Oncol 2016) (2) Functional image biomarker (DWI) (Ongoing) 4. Other than HCC (1) SABR for Cholangiocarcinoma (2017 Tumori, Epub) 5. Development and validation of nomogram for treatment outcome (1) For BCLC stage C disease (International study under ALRT SIG) SIG 6. Immunological biomarkers before and after SABR (1) Tim-3, PD 1, PD L 1 (ongoing)


PURPOSE ¢ To evaluate the survival outcomes and prognostic factors for patients with BCLC stage C HCC after SABR.

MATERIALS &METHODS ¢ A retrospective cohort study ¢ Between December 2007 and July 2015 ¢ BCLC stage C disease ¢ Child-Turcotte-Pugh class A–B



RESULTS ¢ 110 patients with 148 tumors ¢ 1 -year in-field control rate: 76. 9% 3 -year in-field control rate: 66. 4% ¢ 1 -year OS: 49. 6% 3 -year OS: 23. 6%

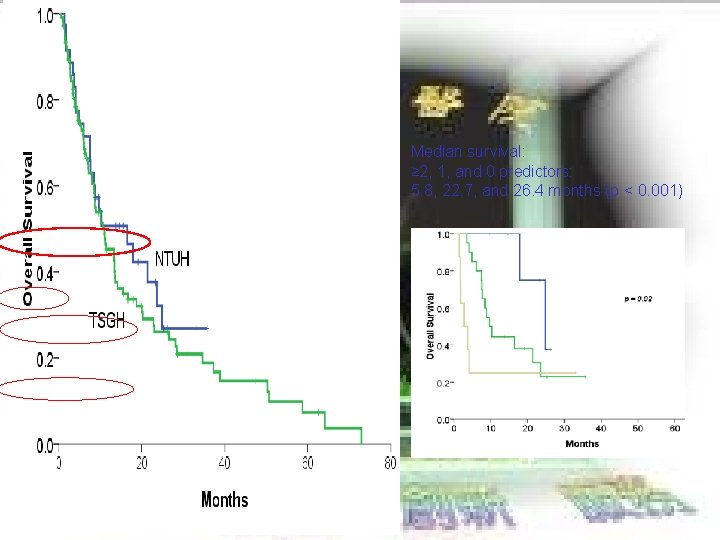
Median survival: ≥ 2, 1, and 0 predictors: 5. 8, 22. 7, and 26. 4 months (p < 0. 001)

EXTERNAL VALIDATION ¢ NTUH 35 cases BCLC stage C Child A/B

EXTERNAL VALIDATION Ø TSGH 110 cases BCLC stage C Child A/B Ø Ø Median survival: ≥ 2, 1, and 0 predictors: 5. 8, 22. 7, and 26. 4 months (p < 0. 001) Ø NTUH 35 cases BCLC stage C Child A/B Median survival: ≥ 2, 1, and 0 predictors: 2. 7, 9. 6, and 24. 8 months (p < 0. 02)

FUTURE WORK ¢ Asia Pacific Primary Liver Cancer Expert Meeting (APPLE) Asia liver radiation therapy special interest group international study ¢ Title: Development and validation of a nomogram for Treatment Outcome of Patients with BCLC stage C HCC after SABR: an international study in Asia

THANK YOU FOR YOUR LISTENING
 State of survival survival of the fittest tweak
State of survival survival of the fittest tweak State of survival survival of the fittest stages
State of survival survival of the fittest stages Per 005 1
Per 005 1 Nrg-gu005
Nrg-gu005 Nom 005 ssa3 2018
Nom 005 ssa3 2018 Csc-005
Csc-005 Hoklas 005
Hoklas 005 Afnor z44-005
Afnor z44-005 Cmbg staffing
Cmbg staffing Sol 003
Sol 003 D.s. n° 005-2012-tr
D.s. n° 005-2012-tr 100000x0.005
100000x0.005 Sigwx chart symbols
Sigwx chart symbols Who prognostic scoring system gtn
Who prognostic scoring system gtn Choriocarcenoma
Choriocarcenoma Emaco regimen
Emaco regimen Palliative prognostic index
Palliative prognostic index Figo prognostic scoring system gtn
Figo prognostic scoring system gtn Van nuys score
Van nuys score Concept map of measurement assessment and evaluation
Concept map of measurement assessment and evaluation