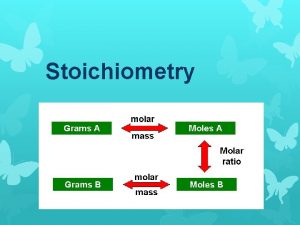
Stoichiometry What is stoichiometry study of quantitative relationships






- Slides: 13

Stoichiometry

What is stoichiometry? • study of quantitative relationships in balanced chemical equation • chemical quations represent chemical reactions

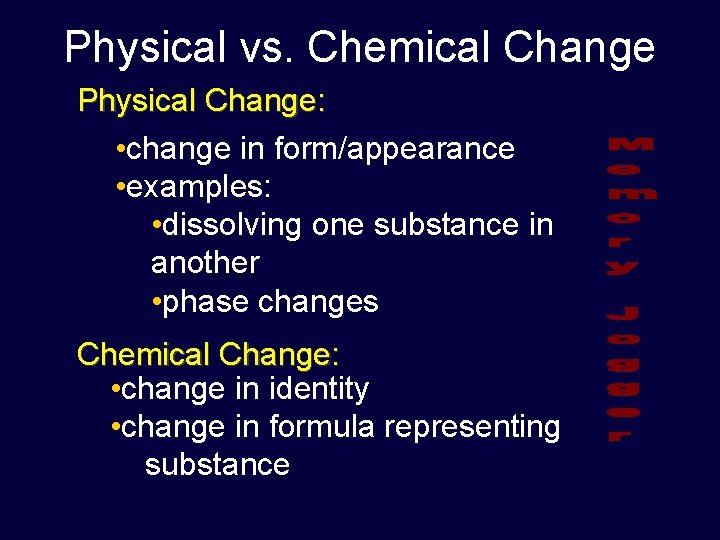
Physical vs. Chemical Change Physical Change: • change in form/appearance • examples: • dissolving one substance in another • phase changes Chemical Change: • change in identity • change in formula representing substance

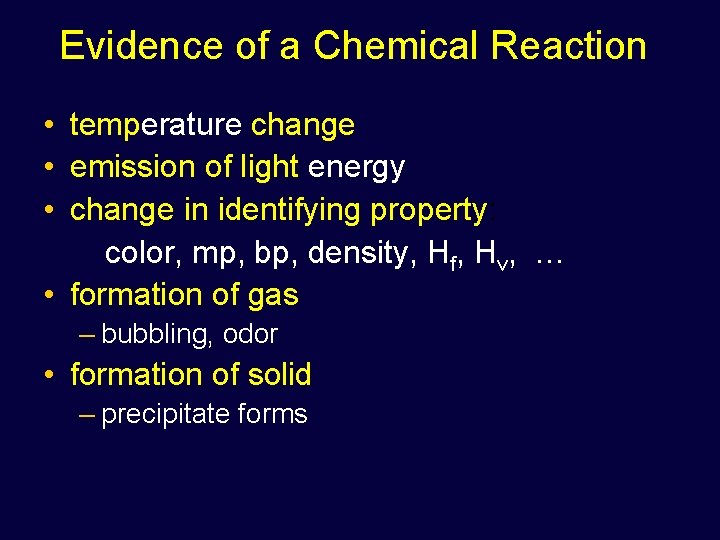
Evidence of a Chemical Reaction • temperature change • emission of light energy • change in identifying property: color, mp, bp, density, Hf, Hv, … • formation of gas – bubbling, odor • formation of solid – precipitate forms

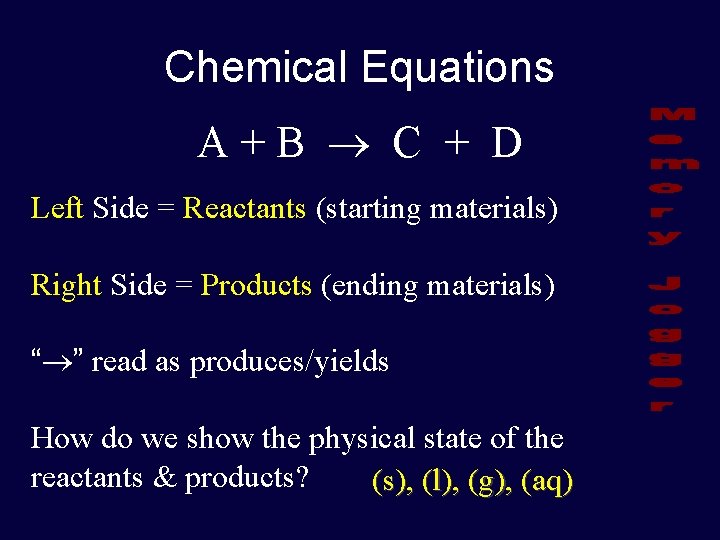
Chemical Equations A+B C + D Left Side = Reactants (starting materials) Right Side = Products (ending materials) “ ” read as produces/yields How do we show the physical state of the reactants & products? (s), (l), (g), (aq)

Law of Conservation of Matter • Matter is neither created nor destroyed in chemical rxns • Mass reactants = Mass products • Chemical bonds in reactants may break; new bonds may form to produce products • # atoms of each element is “constant” – # atoms same on both sides of equation

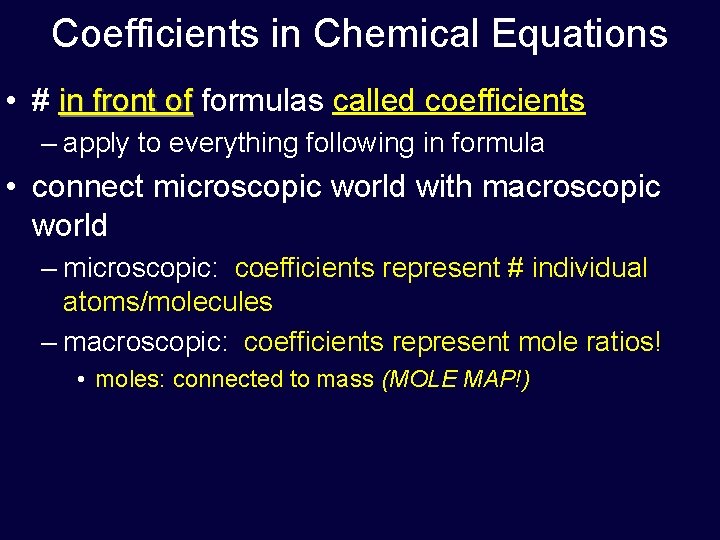
Coefficients in Chemical Equations • # in front of formulas called coefficients – apply to everything following in formula • connect microscopic world with macroscopic world – microscopic: coefficients represent # individual atoms/molecules – macroscopic: coefficients represent mole ratios! • moles: connected to mass (MOLE MAP!)

Writing Chemical Equations • begin with word equation – describes reactants & products – convert words to chemical formulas • next is skeleton equation – replace names of substances with chemical formulas • Balance skeleton equation – balanced equation demonstrates law of conservation of mass

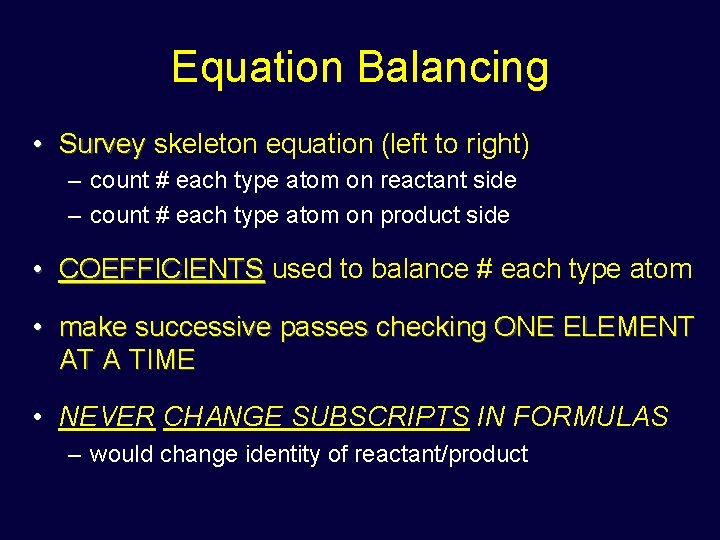
Equation Balancing • Survey skeleton equation (left to right) – count # each type atom on reactant side – count # each type atom on product side • COEFFICIENTS used to balance # each type atom • make successive passes checking ONE ELEMENT AT A TIME • NEVER CHANGE SUBSCRIPTS IN FORMULAS – would change identity of reactant/product

Balanced Equations • coefficients must be in lowest possible ratios • double check your work – always do one last pass to check numbers • use RAP table to keep track of # each element

R Example 1 P 1 Fe 2 2 Fe + O 2 Fe 2 O 3 Fe + 3 O 2 2 Fe 2 O 3 • O’s balanced - Now balance Fe 4 Fe + 3 O 2 2 Fe 2 O 3 • do one last check: 4 Fe, 6 O A ✔ O 3

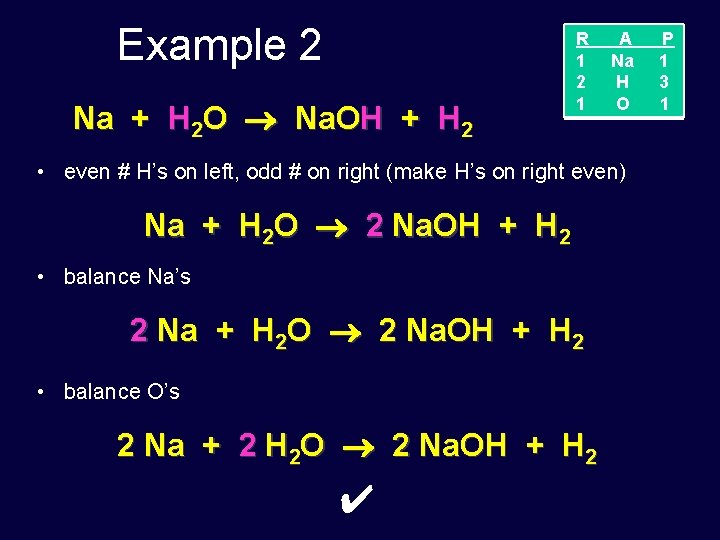
Example 2 Na + H 2 O Na. OH + H 2 R 1 2 1 A Na H O • even # H’s on left, odd # on right (make H’s on right even) Na + H 2 O 2 Na. OH + H 2 • balance Na’s 2 Na + H 2 O 2 Na. OH + H 2 • balance O’s 2 Na + 2 H 2 O 2 Na. OH + H 2 ✔ P 1 3 1

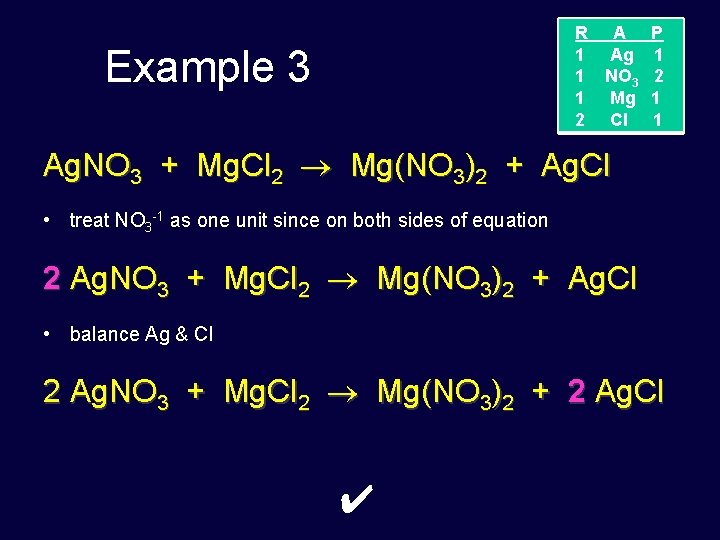
R A 1 Ag 1 NO 3 1 Mg 2 Cl Example 3 P 1 2 1 1 Ag. NO 3 + Mg. Cl 2 Mg(NO 3)2 + Ag. Cl • treat NO 3 -1 as one unit since on both sides of equation 2 Ag. NO 3 + Mg. Cl 2 Mg(NO 3)2 + Ag. Cl • balance Ag & Cl 2 Ag. NO 3 + Mg. Cl 2 Mg(NO 3)2 + 2 Ag. Cl ✔
 Stoichiometry is defined as the quantitative study of
Stoichiometry is defined as the quantitative study of Stoichiometry is defined as the quantitative study of
Stoichiometry is defined as the quantitative study of Quantitative relationships in chemical equations
Quantitative relationships in chemical equations Lesson 2.2 relationships between two quantitative variables
Lesson 2.2 relationships between two quantitative variables Lesson 2.2 relationships between two quantitative variables
Lesson 2.2 relationships between two quantitative variables Chapter 11 stoichiometry test
Chapter 11 stoichiometry test Longitudinal study qualitative or quantitative
Longitudinal study qualitative or quantitative Sociology chapter 15
Sociology chapter 15 Holt mcdougal sociology the study of human relationships
Holt mcdougal sociology the study of human relationships What is conflict theory
What is conflict theory What do ecologists study
What do ecologists study Sociology the study of human relationships
Sociology the study of human relationships What is the most inclusive level of organization
What is the most inclusive level of organization What is case series
What is case series